Synthesis of new 2,5-di(thiophen-2-yl)furan-3-carbonitrile derivatives and investigation of the electrochromic properties of homopolymers and co-polymers with EDOT
Ufuk Abaci*a,
Asli Ustalarb,
Mehmet Yilmaz*b and
H. Yuksel Guneyc
aFord Otosan İhsaniye Automotive Vocational School, Kocaeli University, 41680 Gölcük, Kocaeli, Turkey. E-mail: ufukabaci@kocaeli.edu.tr; Fax: +90 262 435 61 69; Tel: +90 262 435 61 66
bDepartment of Chemistry, Faculty of Arts and Sciences, Kocaeli University, 41380 Umuttepe, Kocaeli, Turkey
cDepartment of Physics, Faculty of Arts and Sciences, Kocaeli University, 41380 Umuttepe, Kocaeli, Turkey
First published on 10th March 2016
Abstract
New 2,5-di(thiophen-2-yl)furan-3-carbonitriles as thiophene–furan–thiophene type monomers (2a and 2b) were synthesized by the oxidation of 2,5-di(thiophen-2-yl)-4,5-dihydrofuran-3-carbonitriles which were prepared by the reaction of 3-oxopropanenitriles with alkenes mediated with manganese(III) acetate using DDQ (2,3-dichloro-5,6-dicyano-p-benzoquinone). The monomers and their copolymers with EDOT were electrochemically polymerized in acetonitrile (AN)/LiClO4. The electrochemical, spectroelectrochemical and morphological properties of the resulting polymer and copolymer films were investigated by cyclic voltammetry, UV-visible spectroscopy and atomic force microscopy. Observed differences in cyclic voltammetry, UV-vis spectroscopy and neutral state absorption (π–π* transition) have been accepted as evidence for the copolymerization. Copolymer films have distinct electrochromic properties according to superposition of the contained polymer properties. In addition the dissolution problem that's causing the instability of poly2b has been fixed with copolymerization. Switch time measurements showed that copolymer films are candidates for ECD applications due to their stability, fast response time and good optical contrast.
1. Introduction
Conducting polyconjugated materials, especially polythiophenes are widely used in transistors,1 photodiodes, LEDs,2–4 sensors,5–7 batteries8,9 and organic photovoltaic devices10–12 due to their optical, electrochemical and structural properties. Although polythiophene,13,14 polypyrrole,15 polyfuran16 and their co-polymers17,18 are well known, polymerization of thiophene–furan–thiophene type monomers and their derivatives have not been studied in detail. Recently, Önal and co-workers reported the anodic polymerization of 2,5-di (2-vinyl) furan and investigated the fluorescent and electrochemical properties of the obtained polymer films. The main restriction to the use of such materials in technology is their insolubility. To enhance their processability, either functionalized monomers or co-monomer mixtures are used to synthesize processable conducting polymers or conducting co-polymer films.19Most of the organic materials exhibit redox states with different electronic (UV-visible) absorption spectra. Where the switching of redox states generates different visible region absorption bands, the material is said to be electrochromic. Color changes are commonly between a transparent (bleached) state and a colored state or between two colored states.20–26
Chemical or electrochemical oxidation of many resonance-stabilized aromatic molecules produces electronically conducting polymers. In their oxidized forms, conducting polymers are doped with counter anions (p-doping) and possess a delocalized π-electron band structure. The energy gap between the highest occupied π electron band (HOMO) and the lowest unoccupied band (LUMO) determining the intrinsic optical properties of these materials. Reduction of conducting polymers removes the electronic conjugation, to give the undoped (neutral) electrically insulating mode.20–22,25,27
It is well known that the optical properties of these materials can be tuned by control the doping process (and/or dedoping). The doping process (oxidation) introduces polarons, which are the major charge carriers. With increasing doping level, bipolaron bands occur in the energy gap. The introduction of new states in the band gap with doping process is the main reason for the changes in the optical properties of conducting polymers and is the main cause for the electrochromism of such materials.20,22,25,28
Electrochemistry has played a significant role in the preparation and characterization of electronically conducting polymers. Electrochemical techniques for the synthesis of conjugated conducting polymers have been considered for a number of optoelectronic and redox properties, such as electrochromism, which is defined as the reversible absorbance/transmittance change in response to an externally applied potential.25,29
Modification of polymer structure and copolymerization are methods to tune electrochromic properties of polymers. In this way copolymerization can lead to an interesting combination of the properties such as fine tuning of the color for electrochromic applications.30,31
Our research group has been studying the synthesis of dihydrofurans by the radical addition of various 1,3-dicarbonyl compounds and 3-oxopropanenitriles with alkenes, alkynes, unsaturated amides, and dienes using manganese(III) acetate.32–36 In this study, 4-phenyl and 4-methyl substituted 2,5-di(thiophen-2-yl)furan-3-carbonitriles (2a and 2b, respectively) has been synthesized from oxidation of suitable 2,5-di(thiophen-2-yl)-4,5-dihydrofuran-3-carbonitriles (1a and 1b, respectively) by DDQ. We comparatively investigated the substituents effect on the electrochromic material properties. Thus, Me (electron donating), Ph (conjugation increasing) and CN (strong electron withdrawing) groups were used as substituents in this study. Homo polymers poly2a and poly2b by anodic polymerization of 2a and 2b have been obtained. Also electrochromic properties of these homo polymer films were investigated. In later stages, copolymers poly2a-co-EDOT and poly2b-co-EDOT were synthesized via anodic polymerization of monomers 2a and 2b with EDOT. Electrochemical and spectroelectrochemical properties of gained these copolymers were investigated via cyclic voltammetry, UV-vis spectroscopy according to obtained morphologies.
PEDOT is the most stable polymer under ambient conditions and it has low solubility in electrolytic medium. On the other hand copolymerization with EDOT improve the stability properties of the polymer film.37–39
The aim of this work is to improve the electrochemical and spectroelectrochemical properties of thiophene–furan type electrochromic materials by copolymerization with EDOT. In this process, polymer film does not undergo electro-dissolution without losing its coloring properties and optical contrast.
2. Experimental section
2.1. General
All chemicals, reagents and solvents are available as commercial products and used in highest purity. Indium tin oxide (ITO) coated glass (Aldrich, 8–12 Ω sq−1) was used as a transparent working electrode. Melting points were determined on an electrothermal capillary melting point apparatus. IR spectra (KBr disc, CHCl3) were obtained with a Matson 1000 FT-IR in the 400–4000 cm−1 range with 4 cm−1 resolution. 1H and 13C NMR spectra were recorded on a Varian Mercury-400 High performance Digital FT-NMR spectrophotometers. The mass spectra were measured with a Waters 2695 Alliance Micromass ZQ (ESI+) LC/MS spectrophotometer. Elemental analyses were performed on a Leco 932 CHNS-O instrument.Electrochemical polymerizations and characterizations were performed by Gamry 750 potentiostat/galvanostat. A three electrode electrochemical cell was used for electrodeposition of monomers. The reference electrode was Ag/Ag+ pseudo reference electrode (Ag wire) and Pt wire as counter electrode. The electrochemical bath of homopolymer film consisted of 0.05 M monomer and 0.1 M LiClO4/acetonitrile (AN) solution. Film depositions were carried out by cyclic voltammetry in the potential range from −0.1 V to 1.0 V (25 mV s−1 scanning rate). Copolymer films were obtained from the solution of 0.05 M monomer (2a or 2b), 20 mM EDOT and 0.1 M LiClO4. Spectroelectrochemical measurements were made using an Agilent 8453 UV-visible spectrophotometer and all of the characterizations were performed in monomer free 0.1 M LiClO4/acetonitrile electrolyte. The morphology of electrochemically deposited films was studied by Park System XE 70 atomic force microscope (AFM). Thin layer chromatography (TLC) was performed on Merck aluminum-packed silica gel plates. Purification of the products was performed by column chromatography on silica gel (Merck silica gel 60, 40–63 μm) or preparative TLC on silica gel of Merck (PF254–366 nm).
2.2. Synthesis of 2,5-di(thiophen-2-yl)-4,5-dihydrofuran-3-carbonitriles (1a and 1b)
A solution of manganese(III) acetate dihydrate (3 mmol, 830 mg) in 15 mL of glacial acetic acid was heated under nitrogen atmosphere at 80 °C until it dissolved. After Mn(OAc)3 dissolved completely, the solution was cooled to 60 °C. A solution of 3-(2-thienyl)-3-oxopropanenitrile40 (2 mmol, 302 mg) and 2-[(E)-2-phenylvinyl]thiophene41,42 (1 mmol, 186 mg) for 1a (or 2-[2-methylvinyl]thiophene (Z/E = 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27) 1 mmol, 124 mg for 1b) in acetic acid was added to this mixture. The reaction was completed when the dark brown color of the solution disappeared (in 30 min). Water was added to this solution and extracted with CHCl3 (3 × 20 mL). The combined organic phases were neutralized with saturated NaHCO3 solution, and dried over anhydrous Na2SO4 and evaporated. Crude products were purified by column chromatography on silica gel or preparative TLC (20 × 20 cm plates, 2 mm thickness) using n-hexane/EtOAc (5
27) 1 mmol, 124 mg for 1b) in acetic acid was added to this mixture. The reaction was completed when the dark brown color of the solution disappeared (in 30 min). Water was added to this solution and extracted with CHCl3 (3 × 20 mL). The combined organic phases were neutralized with saturated NaHCO3 solution, and dried over anhydrous Na2SO4 and evaporated. Crude products were purified by column chromatography on silica gel or preparative TLC (20 × 20 cm plates, 2 mm thickness) using n-hexane/EtOAc (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent.
1) as eluent.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1425, 1205, 913, 714. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.86 (dd, J = 3.6, 1.2 Hz, 1H), 7.51 (dd, J = 4.8, 1.2 Hz, 1H), 7.36 (dd, J = 5.2, 1.2 Hz, 1H), 7.14 (m, 2H), 7.03 (dd, J = 5.2, 3.6 Hz, 1H), 5.48 (d, J = 8.4 Hz, 1H, H-5), 3.52 (m, 1H, H-4), 1.42 (d, J = 6.4 Hz, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 160.9 (C-2), 141.4, 130.4, 130.2, 130.1, 128.3, 127.2, 126.9, 126.6, 116.9 (CN), 88.1 (C-3), 84.3 (C-5), 47.6 (C-4), 18.3 (CH3). LC/MS, m/z (ESI+) 273 (MH+, 100%).
C), 1425, 1205, 913, 714. 1H NMR (400 MHz, CDCl3): δ (ppm) 7.86 (dd, J = 3.6, 1.2 Hz, 1H), 7.51 (dd, J = 4.8, 1.2 Hz, 1H), 7.36 (dd, J = 5.2, 1.2 Hz, 1H), 7.14 (m, 2H), 7.03 (dd, J = 5.2, 3.6 Hz, 1H), 5.48 (d, J = 8.4 Hz, 1H, H-5), 3.52 (m, 1H, H-4), 1.42 (d, J = 6.4 Hz, CH3). 13C NMR (100 MHz, CDCl3): δ (ppm) 160.9 (C-2), 141.4, 130.4, 130.2, 130.1, 128.3, 127.2, 126.9, 126.6, 116.9 (CN), 88.1 (C-3), 84.3 (C-5), 47.6 (C-4), 18.3 (CH3). LC/MS, m/z (ESI+) 273 (MH+, 100%).2.3. Synthesis of 2,5-di(thiophen-2-yl)furan-3-carbonitrile (2a and 2b)
To a solution of 2,5-di(thiophen-2-yl)-4,5-dihydrofuran-3-carbonitriles (1a or 1b) (1 mmol) in CH2Cl2 (20 mL) was added DDQ (1.1 mmol, 0.250 g). The mixture was stirred at room temperature for two days until consume of the dihydrofuran (2) (monitoring by TLC). Water was added to this solution, extracted with CHCl3 (3 × 20 mL) and organic phases were washed with water (3 × 20 mL). The combined organic phases were neutralized with saturated NaHCO3 solution, and dried over anhydrous Na2SO4 and evaporated. Crude products were purified by column chromatography on silica gel using n-hexane/EtOAc (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent.
1) as eluent.
3. Results and discussion
3.1. Synthesis
2,5-Di(thiophen-2-yl)-4,5-dihydrofuran-3-carbonitriles containing 4-phenyl (1a) and 4-methyl (1b) were obtained from the radical reaction of 3-(2-thienyl)-3-oxopropannitrile and corresponding alkenes mediated by manganese(III) acetate (Scheme 1). Then these compounds were converted to 2,5-di(thiophen-2-yl)furan-3-carbonitrile derivatives (2a and 2b) by DDQ in CH2Cl2 at room temperature for two days in good yields (Scheme 2). All new products were characterized by spectroscopic techniques (1H NMR, 13C NMR, LC/MS and IR) and micro analysis.3.2. Cyclic voltammetry
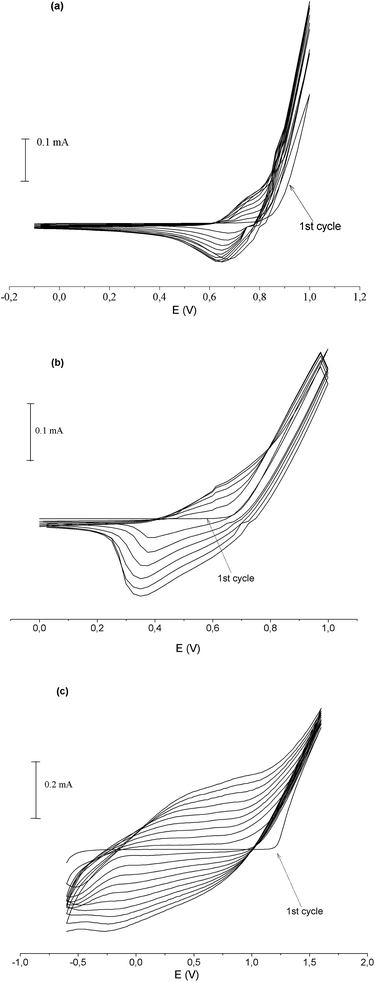
Experiments were carried out in an electrolysis cell equipped with indium doped tin oxide (ITO) coated glass plate as the working, Pt wire counter and Ag/Ag+ reference electrodes.Fig. 1 shows successive cyclic voltammogram of 2a, 2b and EDOT on ITO coated glass, respectively.
It can be seen from Fig. 1a, oxidation of the 2a monomer is about 0.8 V at the first cycle. On subsequent cycles, one anodic and one cathodic peak appeared at the cyclic voltammogram for oxidation and reduction of the polymer respectively. While the oxidation peak of the polymer occurred at about 0.7 V is lower than the monomer oxidation, the reduction peak of the polymer film occurred at about 0.65 V. At the same time, when we look into the successive cycles, the film formation and growth can clearly be observed. On the other hand, for when the same figure investigated for 2b (Fig. 1b), while monomer oxidation occurred about 0.65 V, poly2b shows oxidation and reduction at 0.6 V and 0.38 V respectively. EDOT which were used to form copolymer film has a monomer oxidation about 1.2 V (Fig. 1c). At the same time, with increasing cycle numbers PEDOT has an oxidation about 0.78 V and reduce about 0.45 V respectively.43
The CV curves of poly2a-co-EDOT and poly2b-co-EDOT are shown in Fig. 2. When the CV recorded during the poly2a-co-EDOT formation is investigated (Fig. 2a), it can be seen that oxidation at the first cycle occurred at 0.78 V and the oxidation and reduction of the copolymer film occurred about 0.65 V and 0.4 V respectively. The CV curve of electro polymerization of poly2b-co-EDOT is shown in Fig. 2b. Similarly to poly2a-co-EDOT, poly2b-co-EDOT has a monomer oxidation at 0.78 V at first cycle and the oxidation and reduction potential of the poly2b-co-EDOT occurred about 0.6 V and 0.35 V respectively.
If these results depending on the observations were analyzed, it can be seen that CV curves of copolymers exhibits differences when compared to homopolymer's CV curves. While the monomers have sharp and almost exact redox potentials, oxidation and reduction of copolymers show wide potential dependency for electropolymerization, that is, oxidation and reduction are spread over a wide range of potentials. Nevertheless, when the CV curves recorded during copolymerization are investigated (Fig. 2a and b), it can be seen that the spectral behaviors are similar to the CV curve of PEDOT electropolymerization. Beyond that, when the oxidation/reduction potentials of homopolymers (poly2a, poly2b and PEDOT) and copolymers (poly2a-co-EDOT and poly2b-co-EDOT) are compared; it is clearly can be seen that copolymer formation achieved on the working electrode according to the occurred differences. In addition, the increase in the redox wave current densities implies that the films of conducting copolymers deposited on the ITO working electrode.30,31
3.3. Spectroelectrochemistry
Absorption spectra of poly2a, poly2b and PEDOT are shown in Fig. 3. The absorption spectrum of the poly2a film was measured in the potential range from −0.2 V to 1.2 V (Fig. 3a). The onset energy for the π–π* transition (electronic band gap) was 1.95 eV and λmax was found to be 637 nm. There was a gradual decrease in the peak intensity at 475 nm upon increase in the applied potential, which was accompanied by an increase in the intensity of the peaks at 650 nm due to the formation of charge carrier bands. The appearance of this peak is attributed to the evolution of new electronic bands.19,44 At the potential range −0.2 V to 0.6 V the blue colored film has absorption at 425 nm in the visible range. With the introduction of potentials to higher positive values, at 0.8 V absorption of the film shifts to 650 nm and the color of the film turn to reddish. At 1.2 V absorption of the film shifts to UV range and the film is colorless. Fig. 3a suggests that a significant change in the electronic energy levels, which leads to changes in optical properties is induced by the doping of the poly2a film. | ||
| Fig. 3 Absorption spectra of the homopolymers recorded in the potential range from −0.2 V to 1.2 V in monomer free 0.1 M LiClO4/AN solution: (a) poly2a, (b) poly2b and (c) PEDOT. | ||
Fig. 3b shows the absorption spectrum of poly2b in a monomer free (fresh) solution depending on applied potential. The absorption spectrum of the poly2b film was also measured in the potential range from −0.2 V to 1.2 V, the onset energy for the π–π* transition (Eg) was 1.89 eV and λmax was found to be 657 nm. Undoped neutral form of the polymer represents itself with the 500 nm absorption in the spectrum and this absorption decreased gradually with the increasing potential. As a result of that, new electronic absorption bands (bipolaron) occurred about 670 nm.19,44 Similar to poly2a, poly2b has an absorption in visible range at −0.4 V, moreover at 1.2 V poly2b is colorless and transparent.
Absorption spectra of depending on the applied potential for PEDOT shown in Fig. 3c. π–π* transition (Eg) was 1.61 eV and λmax was found to be 770 nm. As in other two polymers, PEDOT has an absorption in the visible range (blue color) for negative potentials, with the introduction to positive potentials PEDOT film passes through transparent mode.
Absorption spectra of copolymer films are given in Fig. 4a and b. Poly2a-co-EDOT has a π–π* transition (Eg) 1.69 eV and λmax was found to be 736 nm (Fig. 4a). When the absorption properties of poly2a and PEDOT investigated, it can be concluded that the dominant character of PEDOT represents itself in the spectra. While at negative potential (−0.2 V) copolymer film is in its colored form and has an absorption around 560 nm, with the increasing potentials towards the positive potentials copolymer film passes trough transmissive mode with no absorption in visible range.
 | ||
| Fig. 4 Absorption spectra of the copolymer films recorded in the potential range from −0.2 V to 1.2 V in monomer free 0.1 M LiClO4/AN solution: (a) poly2a-co-EDOT and (b) poly2b-co-EDOT. | ||
Fig. 4b shows absorption spectra of poly2b-co-EDOT depending on applied potential. π–π* transition (Eg) of poly2b-co-EDOT was found to be 1.67 eV at λmax 744 nm. Different from the poly2a-co-EDOT, the spectral behavior of poly2b-co-EDOT represents the properties of both polymers (poly2b and PEDOT). If we take into account absorption spectrum of poly2b and PEDOT (Fig. 3b and c), it can be seen that, while poly2b has neutral form absorption at 500 nm, PEDOT represents its neutral (undoped) absorption at 600 nm. Based on the light of these informations, when we deal with the absorption spectrum of poly2b-co-EDOT again, it can be concluded that the spectrum represents the superposition of both polymers. At the same time, like all of the other films, with the introduction to positive potentials absorption of poly2b-co-EDOT shift from visible range of infrared and film gets its transparent mode.
If all UV-vis spectra of homo and copolymers were examined; π–π* transitions (Eg) of poly2a, poly2b and PEDOT found to be 1.95 eV, 1.89 eV and 1.61 eV respectively. In addition, neutral form absorptions of the films, for the same order, are determined as 475 nm, 500 nm and 770 nm respectively. On the other hand, at the measurements taken for copolymers; poly2a-co-EDOT has a π–π* transition (Eg) at 1.69 eV (λmax = 736 nm) and for poly2b-co-EDOT π–π* transition (Eg) is 1.67 eV (λmax = 744 nm). These values are all together shown in Table 1 for comparison. The observed differences in Eg values and neutral form absorptions wavelengths are other evidences to conclude that copolymerization is successfully achieved.30 In addition, the decreasing bandgap of the copolymer films can be attributed to more EDOT units incorporation into the copolymer chains.39,45,46 The λmax values of the copolymers were red-shifted compared to that of the homopolymer, which is due to increase in conjugation length and the influence of high electron density resulting from the incorporation of EDOT into 2a and 2b units. As the amount of EDOT increases in the copolymer, the maximum wavelength of the π–π* transition increases and the electronic band gaps for the copolymers decrease.47
| Eg (eV) | λmax (nm) | |
|---|---|---|
| Poly2a | 1.95 | 637 |
| Poly2b | 1.89 | 657 |
| PEDOT | 1.61 | 770 |
| Poly2a-co-EDOT | 1.69 | 736 |
| Poly2b-co-EDOT | 1.67 | 744 |
Fig. 5 shows the transmittance spectra for the homopolymer films at different applied potentials. As can be seen from the Fig. 5a the maximum conditions at 500 nm, poly2a has 29.41% and 69.27% for the bleached and the colored states respectively. In addition, starting from this point, it can be calculated that the optical contrast (ΔT%) of the poly2a film as 39.86% at 500 nm. On the other hand, poly2b shows its maximum contrast at 500 nm (Fig. 5b) and while for bleached state film has 81.93% transmittance, for colored state transmittance found to be 48.6%. Therefore, optical contrast of poly2b calculated as 33.33% for 500 nm at working potentials. When these results compared with the literature, it can be concluded that optical contrast of the homopolymers at the same range with furan derivative polymer films.19
 | ||
| Fig. 5 Transmittance spectra of the homopolymer films at −0.2 V and 1.2 V in monomer free 0.1 M LiClO4/AN solution: (a) poly2a, (b) poly2b and (c) PEDOT. | ||
Transmittance spectra of copolymers taken between maximum working potentials (−0.2 V/1.2 V) in monomer free solution are shown in Fig. 6. If we consider again the absorption spectrum of poly2a-co-EDOT; PEDOT was the dominant polymer for spectral behavior (Fig. 6a). Furthermore, when the transmittance spectrum of poly2a-co-EDOT is analyzed in the same manner, it can be seen that PEDOT is again dominant for spectral behavior. The optical contrast of the copolymer film was calculated as 34.17% at 520 nm and for this calculation transmittances for the colored and bleached states were measured 23.22% and 57.39% respectively. Fig. 6b shows the transmittance spectrum of poly2b-co-EDOT depending on applied potentials. Poly2b-co-EDOT which represents the superposition of its constituent polymers has maximum transmittance at 485 nm for 1.2 V. Spectrum of poly2b-co-EDOT has a peak and associated shoulder depending on the properties of poly2b and PEDOT and for the maximum situations (at 485 nm), copolymer film has transmittances 35.28% and 65.83% for colored (−0.2 V) and bleached (1.2 V) modes, respectively. Depending on these measurements, optical contrast (ΔT%) of poly2b-co-EDOT has been calculated as 30.55%.
 | ||
| Fig. 6 Transmittance spectra of the comopolymer films at −0.2 V and 1.2 V in monomer free 0.1 M LiClO4/AN solution: (a) poly2a-co-EDOT and (b) poly2b-co-EDOT. | ||
If the films obtained from copolymerization of 2a and 2b with EDOT as well as the homopolymer films of poly2a and poly2b were analyzed, it can be seen that all of the films have ΔT% values around 30%. The spectral behavior of copolymer films shows that the obtained spectra are the superposition of both polymers.39,47 At the same time, when all of the results take into account it can be concluded that, obtained homo and copolymers can be used for ECD applications with their spectroelectrochromic properties.
3.4. Electrochromic switching
The ability of a polymer to switch without delay and exhibit a sharp color change is very significant. Double potential step chronoamperometry was carried out to determine the switch time. The potential was stepped from −0.2 V to 1.2 V with a residence time of 5 s in a monomer free solution. During the experiment, the % transmittance at the wavelength of maximum contrast was measured by a UV-vis spectrophotometer. Fig. 7 and 8 show the transmittance–time profile of the homo and copolymer films recorded during double step spectrochronoamperometry.As seen in Fig. 7a, poly2a has 0.9 s bleaching time and 1 s to be colored with a reasonable optical contrast (∼40%).
On the other hand, Fig. 7b shows transmittance–time profile of poly2b. While the switch time of the film is similar to poly2a, poly2b losing its stability for the increasing number of potential steps. This situation could be attributed to degradation and the dissolution problem of the polymer film. These indicate some electrochromic properties depleting after consecutive switching, which is normal in electrochromic conducting polymers.39
Fig. 8a and b, shows the switch time measurements for poly2a-co-EDOT and poly2b-co-EDOT respectively. While bleaching time was measured 1.21 s for poly2a-co-EDOT coloring time was 1.38 s for the same copolymer film (Fig. 8a).
On the other hand, response time of poly2b-co-EDOT system for coloration and bleaching was measured 1.05 s and 1.2 s respectively. In addition, the main point to be considered is increased stability of the film. Stability of poly2b homopolymer was weak depending on the degradation and dissolution of the film. Here it can be said that, the copolymerization of 2b with EDOT has an advantage on increasing optical stability of the film with the preservation properties of poly2b. This result could be attributed to morphological differences of copolymer film comparing to homopolymer film of poly2b.
Switching speed of electrochromic materials can be strongly interacted by the surface morphology and electroactivity of film, film shows rough and porous morphology or high electroactivity which benefit ions penetration exhibiting fast switching speed.38,48 Effect of morphological changes on switch time can be ascribed to the introduction of EDOT units into the polymer backbone.30
3.5. Morphology
The morphology of electrochemically deposited homopolymer and copolymer films were studied by AFM and images are given in Fig. 9 and 10. | ||
| Fig. 10 Atomic force microscopy images of (a) poly2a-co-EDOT, (b) poly2b-co-EDOT, (c) 3D poly2a-co-EDOT and (d) 3D poly2b-co-EDOT. | ||
When morphologies of homo and copolymer films are investigated it can be seen that typical rough films with small grains and spots were obtained via electropolymerization method.49–51 If the film roughness were taken into account; while poly2a has 40.74 nm RMS (root mean surface) roughness, poly2b has a roughness of 20.53 nm. On the other hand, the roughness of poly2a-co-EDOT and poly2b-co-EDOT are 73.2 nm 57.35 nm, respectively. These results show that the roughnesses of all films are higher than furan type polymer films52 which may lead to better film stability and optical response.
The roughness of the electrochemically obtained films have an important role for the doping/dedoping process of the system.49,53 Increasing film roughness and porosity facilitate the ions insertion extraction to the film and reducing the electrode impedance with increasing film area and as a result of these effects, oxidation/reduction process of the film becomes easier.38,48 In this context, when electro and spectroelectrochemical properties of the homo and copolymer films are investigated, it is easy to explain the results in a meaningful way: the films with lower roughness and porosity have relatively more difficult doping/dedoping process and as a result of that inappropriate, low stable films (poly2b) are obtained for ECD applications. On the contrary, copolymer obtained from the electropolymerization of 2b and EDOT (poly2b-co-EDOT) has higher roughness. As a result of that, doping/dedoping process is easier and therefore the film becomes appropriate for ECD applications with the inherit spectroelectrochemical properties of poly2b.
4. Conclusions
A new CN and Ph functionalized thiophene–furan–thiophen type of monomers were synthesized and characterized by spectroscopic techniques. The films of homopolymers were obtained electrochemically in AN using 0.1 M LiClO4 as supporting electrolyte. In addition, a new copolymers based on 2a or 2b and EDOT were synthesized by electrochemical oxidation of the monomer mixture 2a or 2b/EDOT in the solution of 0.1 M LiClO4/AN.Electrochemical, electrochromic and morphological properties of the homopolymer and copolymer films were investigated by cyclic voltammetry, UV-vis spectroscopy and atomic force microscopy, respectively. The contrast was determined as the difference between T% in the reduced and oxidized forms and measured 48.53% and 33.33% for poly2a and poly2b respectively. On the other hand copolymer films of poly2a-co-EDOT shows 34.14% and poly2b-co-EDOT 30.55% ΔT% values for the working potential range. While the band gap of the poly2a film was found as 1.95 eV, π–π* transition of poly2b was found as 1.89 eV. In addition band gaps of poly2a-co-EDOT and poly2b-co-EDOT were calculated 1.69 eV and 1.67 eV respectively. Although there are a lot of work in the literature for electrochromic materials, thiophene–furan–thiophene type monomers and their derivatives have not been studied in detail. The main problem to the use of such materials in technology is their insolubility and instability. Switch time measurements showed that, copolymerization of this TFT type monomer with EDOT improve the film stability to work with acceptable optic contrast values. As a result of all analyses, copolymer films of 2a and 2b with EDOT are promising candidates for ECD applications.
Acknowledgements
The authors gratefully thank the Kocaeli University (BAP 2007/73, 2010/57, 2012/28) Science Research Foundations for financial support. Also. A. Ustalar thanks to TUBITAK for doctoral fellowship.References
- C. Shu and M. S. Wrighton, J. Phys. Chem., 1988, 92, 5221 CrossRef CAS; G. Horowitz, Adv. Mater., 1998, 10, 365 CrossRef.
- G. Gustafsson, Y. Cao, G. M. Treacy, F. Klavetter, N. Colaneri and A. J. Heeger, Nature, 1992, 357, 477 CrossRef CAS.
- P. Barta, J. Santra and M. Zagorska, Synth. Met., 1998, 94, 119 CrossRef CAS.
- Y. Liu, Y. Xu and D. Zhu, Macromol. Chem. Phys., 2001, 202, 1010 CrossRef CAS.
- L. S. Hwang, J. M. Ko, H. W. Rhee and C. Y. Kim, Synth. Met., 1993, 55, 3671 CrossRef.
- J. M. Slater, E. J. Watt, J. Freeman, J. P. May and D. J. Weirm, Analyst, 1992, 117, 1265 RSC.
- P. N. Bartlett and S. K. Ling Chung, Sens. Actuators, 1989, 19, 141 CrossRef CAS.
- R. Bittihm, G. Ely, F. Woeffier, H. Munstedt, H. Narmann and D. Naegele, Makromol. Chem., Macromol. Symp., 1987, 8, 51 CrossRef.
- M. Mermillod, J. Tanguy and F. Petiot, J. Electrochem. Soc., 1986, 133, 1073 CrossRef.
- S. Gunes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324 CrossRef PubMed.
- J. C. Bijleveld, A. P. Zoombelt, S. G. J. Mathijssen, M. M. Wienk, M. Turbiez, D. M. Leeuw and R. A. J. Janssen, J. Am. Chem. Soc., 2009, 131, 16616 CrossRef CAS PubMed.
- Q. Zhang, A. Cirpan and T. P. Russell, Macromolecules, 2009, 42, 1079 CrossRef CAS.
- J. Roncali, Chem. Rev., 1997, 97, 173 CrossRef CAS PubMed.
- F. Estrany, R. Oliver, P.-L. Cabot and E. Brillas, Eur. Polym. J., 2006, 42, 563 CrossRef CAS.
- A. F. Diaz and K. K. Kanazawa, J. Chem. Soc., Chem. Commun., 1979, 14, 635 RSC.
- G. Tourillon and F. Garnier, J. Electroanal. Chem., 1982, 135, 173 CrossRef CAS.
- A. G. Shilabin and A. A. Entezami, Eur. Polym. J., 2000, 36, 2005 CrossRef CAS.
- J. P. Ferrais and T. R. Hanlon, Polymer, 1989, 30, 1319 CrossRef.
- M. İçli, A. Cihaner and A. M. Önal, Electrochim. Acta, 2007, 52, 8039 CrossRef.
- R. J. Mortimer, Electrochim. Acta, 1999, 44, 2971 CrossRef CAS.
- R. J. Mortimer, A. L. Dyer and J. R. Reynolds, Displays, 2006, 27, 2 CrossRef CAS.
- P. R. Somani and S. Radhakrishnan, Mater. Chem. Phys., 2002, 77, 117 CrossRef.
- A. Malinauskas and R. Holze, Synth. Met., 1998, 97, 31 CrossRef CAS.
- L. M. Huang, C. H. Chen, T. C. Wen and A. Gopalan, Electrochim. Acta, 2006, 51, 2756 CrossRef CAS.
- P. M. S. Monk, R. J. Mortimer and D. R. Rosseinsky, Electrochromism and electrochromic devices, Cambridge University Pres, Cambridge, 1st edn, 2007 Search PubMed.
- B. Scrosati, Applications of electroactive polymers, Chapman and Hall, London, 1st edn, 1993 Search PubMed.
- A. J. Heeger, N. S. Sariciftci and E. B. Namads, Semiconducting and metallic polymers, Oxford University Pres, New York, 1st edn, 2010 Search PubMed.
- R. J. Mortimer, Chem. Soc. Rev., 1997, 26, 147 RSC.
- M. Ak, V. Gancheva, L. Terlemezyan, C. Tanyeli and L. Toppare, Eur. Polym. J., 2008, 44, 2567 CrossRef CAS.
- B. Wang, J. Zhao, R. Liu, J. Liu and Q. He, Sol. Energy Mater. Sol. Cells, 2011, 95, 1867–1874 CrossRef CAS.
- B. Yigitsoy, S. Varis, C. Tanyeli, I. M. Akhmedov and L. Toppare, Electrochim. Acta, 2007, 52, 6561 CrossRef CAS.
- M. Yilmaz and A. T. Pekel, Synth. Commun., 2001, 31, 3871 CrossRef CAS.
- O. Alagoz, M. Yilmaz and A. T. Pekel, Synth. Commun., 2006, 36, 1005 CrossRef CAS.
- M. Yilmaz, Tetrahedron, 2011, 67, 8255 CrossRef CAS.
- M. Yilmaz and A. T. Pekel, J. Fluorine Chem., 2011, 132, 628 CrossRef CAS.
- E. Bicer, M. Yilmaz, M. Karatas and A. T. Pekel, Helv. Chim. Acta, 2012, 95, 795 CrossRef CAS.
- A. S. Saraç, G. Sonmez and F. Ç. Cebeci, J. Appl. Electrochem., 2003, 33, 295 CrossRef.
- Y. J. Tao, H. F. Cheng, W. W. Zheng, Z. Y. Zhang and D. Q. Liu, Synth. Met., 2012, 162, 728 CrossRef CAS.
- Z. Y. Zhang, Y. J. Tao, X. Q. Xu, Y. J. Zhou, H. F. Cheng and W. W. Zheng, J. Appl. Polym. Sci., 2013, 129, 1506 CrossRef CAS.
- K. Drauz, A. Kleemann and E. Wolf-Heuss, US Pat., 4, 728, 743, March 1 1988.
- A. J. Fry, M. Allukian and A. D. Williams, Tetrahedron, 2002, 58, 4411 CrossRef CAS.
- E. Maccarone, A. Mamo, G. Perrini and M. J. Torre, J. Chem. Soc., Perkin Trans. 2, 1981, 324 RSC.
- K. Kadac, A. Nowaczyk and J. Nowaczyk, Synth. Met., 2015, 206, 145 CrossRef CAS.
- E. Brillas, J. Carrasco, G. Anton and T. F. Otero, Synth. Met., 1999, 101, 25 CrossRef CAS.
- S. Varis, M. Ak, I. M. Akhmedov, C. Tanyeli and L. Toppare, J. Electroanal. Chem., 2007, 603, 8 CrossRef CAS.
- E. Yildiz, P. Camurlu, C. Tanyeli, I. Akhmedov and L. Toppare, J. Electroanal. Chem., 2008, 612, 247 CrossRef CAS.
- M. Ak, H. Çetişli and L. Toppare, Colloid Polym. Sci., 2013, 291, 767 CAS.
- U. Abaci, H. Y. Guney and U. Kadiroglu, Electrochim. Acta, 2013, 96, 214 CrossRef CAS.
- T. N. Wei, H. O. Kok, T. L. Ting, J. C. Soo and X. Jianwei, J. Mater. Chem. C, 2015, 3, 5589 RSC.
- K. D. O'Neil and O. A. Semenikhin, J. Phys. Chem. C, 2007, 111, 14823 Search PubMed.
- L. F. O. Martins, R. M. Q. Mello, M. L. Sartorelli, I. A. Hümmelgen and A. A. Pasa, Phys. Status Solidi A, 2004, 201, 902 CrossRef CAS.
- D. Sheberla, S. Patra, Y. H. Wijsboom, S. Sharma, Y. Sheynin, A. Haj-Yahina, A. H. Barak, O. Gidron and M. Bendikov, Chem. Sci., 2015, 6, 360 RSC.
- W. T. Neo, Q. Ye, T. T. Lin, S. J. Chua and J. Xu, Sol. Energy Mater. Sol. Cells, 2015, 136, 92 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |