Correlating the surface structure and hydration of a γ-Al2O3 support with the Run (n = 1–4) cluster adsorption behavior: a density functional theory study†
J. Yanga,
H. Wangb,
X. Zhaob,
Y. L. Lib and
W. L. Fan*a
aKey Laboratory for Colloid and Interface Chemistry of State Educating Ministry, School of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, China. E-mail: fwl@sdu.edu.cn; Fax: +86-531-88364864; Tel: +86-531-88366330
bState Key Laboratory of Crystal Materials, Shandong University, Jinan 250100, China
First published on 13th April 2016
Abstract
The adsorption and nucleation behavior of Run (n = 1–4) clusters deposited on absolutely dehydrated and hydroxyl-modified γ-Al2O3 (100) and (110) surfaces were studied using density functional theory slab calculations. The results indicated that the adsorption process was strongly sensitive to cluster size and surface structure, with deformation and metal–support interactions apparently related. A single Ru atom preferred to adsorb on the (100) surface with small deformation energies, while small interaction energies led to the adsorption of Ru4 clusters on the (110) surface. When the surface was dehydrated, the adsorption of Run (n = 2–4) clusters on the (110) surface was substantially more stable than that on the (100) surface. The stronger acceptor and almost equal donor sites present on the dehydrated (110) surface increased the bidirectional electron transfer between the clusters and surface sites, resulting in lower adsorption and interaction energies. When the surface was hydrated, the introduction of hydroxyl groups lowered the Run (n = 2–4) clusters' adsorption ability on the hydrated (110) surface by decreasing the surface acidity and basicity, thereby weakening the driving force underlying electron transfer. However, for single Ru atoms, the opposite behavior was observed. In this case, the surface hydroxyl groups increased the stability of the adsorption of the Run (n = 1–4) clusters on the hydrated (100) surface as surface H acted as an adsorption site, receiving an electron from the Ru atom because of its strong Lewis acidity. Further, the support can stabilize the Run (n = 2–4) clusters by decreasing the binding energies of supported configurations lower than the corresponding gas phase. And the nucleation of Run clusters on all surfaces is thermodynamically favourable. The hydration of the (110) surface facilitates agglomeration of Ru4 clusters, while it is suppressed on the (100) surface. These results are relevant to understanding the interaction between surfaces and clusters.
1. Introduction
Economically sustainable development has driven the development of heterogeneous catalysis in the fields of oil refining,1 exhaust gas treatment,2–4 and organic degradation5,6 to solve the energy crisis and environmental pollution problems. Heterogeneous catalysts consisting of metal clusters supported on metal oxides are widely recognized and very attractive because of their highly disperse active phases, resistance to loss of activity, and unique interface sites.7–10 Conventionally, the support was believed to function only as an inert matrix, contributing to the dispersion and stability of the metal clusters, whereas the metal was the active phase during catalysis. However, by combining elegant experiments and state-of-the-art theory, researchers have shown that the chemical and physical properties of clusters and supports are changed by the interactions that occur when metal clusters are deposited and grown on oxide supports.11–13 Consequently, the adsorption behavior, catalytic reactivity and catalytic selectivity can also be influenced, sometimes in unexpected ways.14–23 For example, the bond length of Au12 clusters increased after being supported on the (100) surface of MgO, increasing the ability to adsorb CO and O2.11,24 Rodríguez et al.22 suggested that when Ni covers only a small portion of the CeO2 (111) surface, it exists in the +2 state, thereby inducing high selectivity for water–gas shift reactions. In contrast, however, for large coverage, Ni is present in a lower oxidation state, leading to the production of CH4. These phenomena revealed that heterogeneous catalysts are complex and that it is necessary to systematically study metal–support interactions. Recently, Raróg-Pilecka et al.25 generated Ru/Al2O3 with higher turnover frequencies (TOFs) than Ru/MgAl2O4, Ru/MgO, and Ru/C for COX methanation. Previously, Akane et al.14 observed that minimizing the interactions between Ru nanoparticles and the Al2O3 support led to high activity for ammonia synthesis. Unfortunately, the interaction mechanism was a difficult puzzle to solve. Therefore, theoretical studies of the interactions between Ru and Al2O3 should constitute an integral part of developing heterogeneous catalysts.Currently, new discoveries are being made based on ongoing phenomenological studies. Recently, Kwak et al.26 achieved a high CO yield via CO2 reduction using low Ru loading on Al2O3 (≤0.5 wt%), whereas CH4 formed when high Ru loading was used. These results indicated that the selectivity of CO2 reduction was sensitive to cluster size. However, the original structure of Ru/Al2O3 was unknown. To the best of our knowledge, no theoretical study focused on the correlation between cluster size and metal–support interactions in Ru/Al2O3 has yet been published. It is well known that the exposed surface plays a pivotal role in these interactions and the subsequent reaction performance, which can be traced to the various geometries and electronic structures of the surface atoms in different surfaces.27–30 For example, Wei et al.30 suggested that the Ru/TiO2 (101) surface had a higher activity than the Ru/TiO2 (001) surface for CO2 methanation because of the stronger interactions between Ru clusters and the TiO2 (101) surface. Thus, a detailed analysis relating surface activity and such interactions is needed. In addition, the surface environment, which frequently contains hydroxyl groups,31 should trigger the electronic redistribution of atoms and surface reconstruction, thereby altering the active sites.32 Layman et al.33 noted that the absorbance features of CO were red-shifted on hydrated 5 wt% Ru/Al2O3 compared to the dry condition, suggesting that water slightly altered the surface properties and thereby influenced the reactant adsorption. Behm et al.34 recently observed that the presence of water decreased the mean Ru particle size in Ru/Al2O3 catalysts concomitant with decreased CO2 dissociation activity. Therefore, the influence of water should not be neglected when in heterogeneous catalysis of this type. Therefore, connecting the quantum calculations with experiment results and determining the bases for these experimental observations to design an appropriate synthetic method for highly efficient catalysts are worthwhile activities.
In this paper, the structure sensitivity of γ-Al2O3 was studied by investigating the adsorption of Run (n = 1–4) clusters on dehydrated and hydrated (110) and (100) surfaces using density functional theory (DFT) calculations. The results showed that the Run adsorption process was strongly sensitive to the particle size and surface structure. Our work aimed to gain insights into the cluster size, surface sites, and effects of hydroxyl groups on adsorption behavior and provide a theoretical basis for the design of tailor-made catalysts. The paper is organized as follows: in the second section, the calculation methods and models are briefly described. In the third section, the Run (n = 1–4) cluster adsorption on dehydrated and hydrated γ-Al2O3 surfaces is presented and discussed. Finally, in the fourth section, we summarize the main conclusions of our study.
2. Methods and models
All of the periodic DFT35 calculations were conducted in the Cambridge Sequential Total Energy Package (CASTEP) program.12 The Perdew–Burke–Ernzerhof (PBE) functional within the generalized gradient approximation (GGA)28 was chosen to describe the exchange correlation energy. A plane wave basis set was implemented to expand the electron wave functions, and the interaction between ion cores and valence electrons was handled with ultrasoft pseudopotentials. For Ru, the 4s, 4p, 4d, and 5s states were treated as valence states, whereas for Al, the 3s and 3p states were treated as the valence states. A cutoff energy of 340 eV was used throughout our calculations. The convergence test for the cutoff energy is included in the ESI (Fig. S1†). The Monkhorst–Pack scheme k-point grids were set: 4 × 3 × 3 for the Al2O3 bulk, 3 × 3 × 1 for the (110) surface, and 2 × 3 × 1 for the (100) surface. The maximum force for the convergence criteria of geometry optimization was chosen as 0.03 eV Å−1. The structure was relaxed until the total energy converged to less than 1.0 × 10−5 eV per atom, the stress was less than 0.05 GPa, and the displacement was less than 0.001 Å. For the energy calculation, a value of 1.0 × 10−6 was set for self-consistent field (SCF) tolerance. To improve the computational performance, a smearing of 0.1 eV was used. The Mulliken charge population and charge density difference for involved atoms was used to study the interactions between metal clusters and supports. Calculations show that the spin-polarized of Run cluster has little effect on the adsorption energies and charge transfer for Run cluster adsorption on Al2O3 support (Tables S3–S7, ESI†). Although the absolute values of the Mulliken charge have no actual meaning, the relative values could facilitate understanding the redistribution of charges and the strength of the forming bonds.The non-spinel Al2O3 model was adopted to construct the surfaces in our work because the penta-Al sites on the (100) surface have detected experimentally.36,37 The same unit cell size was used for the study of Ru deposition, as in previous works.38,39 Based on our test (Fig. S3 and Table S1†) and previous report,31 O4c–Al3c–2O2c terminated surfaces are chosen to model the (110) surface, meanwhile, Al5c terminated surfaces are for (100) surface. This calculation yielded the lattice parameters of the bulk crystal structure: a = 5.60 Å, b = 8.43 Å, and c = 8.08 Å; α = β = γ = 90°. The (110) surface was modeled using a unit cell (1 × 1) with dimensions of 8.43 Å × 8.08 Å × 19.18 Å in six layers. In the calculations, the bottom atomic layers were frozen, but the four topmost layers and Run were allowed to relax. The (100) surface was modeled by a supercell (2 × 1) with dimensions of 11.20 Å × 8.43 Å × 20.45 Å in ten layers. Because they were differentiated from the (110) surface, the bottom four layers were fixed. The hydrated surface was built by adsorbing H2O, yielding a surface-adsorbed hydroxyl radical and a surface-terminated hydroxyl radical. The vacuum region was set to 12 Å in the z direction to separate the slabs; this distance is sufficient to shield the self-interaction of the periodic boundary conditions according to the convergence test presented in Fig. S2.†
As a standard for the relative stability of isolated Run (n = 2–4) clusters, we took the average binding energy, Ebind(Run), of a cluster, typically defined as follows:
| Ebind(Run) = [E(Run) − n × E(Ru)]/n | (1) |
To evaluate the stability of the adsorption structure, the adsorption energy (Eads) of a Run (n = 1–4) cluster deposited on the substrate was defined as follows:
| Eads = E(Run/γ-Al2O3) − E(γ-Al2O3) − E(Run) | (2) |
| Eint = E(Run/γ-Al2O3) − E(γ-Al2O3)′ − E(Run)′ | (3) |
| Edef,surface = E(γ-Al2O3)′ − E(γ-Al2O3) | (4) |
Similarly, Edef,Run is the energy difference between free clusters and adsorbed clusters, as follows:
| Edef,Run = E(Run)′ − E(Run) | (5) |
To explore the influence of surface structure and hydration on the stability of Run (n = 1–4) cluster, the average binding energy, Ebind(Run/γ-Al2O3), of supported Run cluster on four different surfaces are calculated as follows:
| Ebind(Run/γ-Al2O3) = [E(Run/γ-Al2O3) − n × E(Ru) − E(γ-Al2O3)]/n | (6) |
Based on the most preferable adsorption configurations of Run (n = 1–4) clusters on γ-Al2O3 surface, we further investigated the nucleation of Run cluster as follows:
| Enuc = E(Run/γ-Al2O3) + E(γ-Al2O3) − E(Run−1/γ-Al2O3) − E(Ru/γ-Al2O3) | (7) |
3. Results and discussion
3.1 Gas-phase clusters
To understand the adsorption of clusters on the substrate, we first explored the geometries and energies of isolated Run (n = 2–4) clusters. The geometries of Run (n = 2–4) clusters and bulk material are obtained using the methods described above and are presented in Fig. 1, which shows the one-dimensional (1D), two-dimensional (2D), and three-dimensional (3D) structures of Run (n = 2–4) clusters. The stable geometries are summarized in Table 1. The Ru–Ru bond length in the linear Ru2 cluster is 1.96 Å, and the formation process is exothermic by 3.81 eV. For the Ru3 cluster, the average bond length is 2.28 Å in a triangle configuration, and the formation process of cluster is exothermic by 4.20 eV. For the Ru4 cluster, the planar and spatial configurations were both investigated. Although the 3D tetrahedral configuration is less stable than the 2D planar one, the tetrahedral structure, as the smallest unit, can be used to examine the metal–metal and metal–support interactions. Thus, we employed the tetrahedral configuration in the subsequent study.| n | Geometry | d(Ru–Ru) (Å) | Ebind(Run) (eV) |
|---|---|---|---|
| 2 | D∞h | 1.96 | −3.81 |
| 3 | D3h | 2.28 | −4.20 |
| 4 | D4h | 2.21 | −5.20 |
| 4 | Td | 2.42 | −5.00 |
As listed in Table 1, the Ru–Ru bond distance generally tends to increase with atomic coordination. However, the Ru–Ru bond distance of the clusters is smaller than that in the bulk (dRu–Ru = 2.66 Å). Additionally, the binding energies of Run (n = 2–4) clusters decrease with the cluster size (except for the planar Ru4 cluster), indicating that the Ru4 tetrahedral cluster is the most stable.
3.2 γ-Al2O3 surface structures
The relaxed structure of the dehydrated γ-Al2O3 (110) surface is shown in Fig. 2 (D(110)) and consists of 3-fold-Al, 4-fold-coordinated Al, 2-fold-coordinated O, and 3-fold-coordinated O distributed across the uneven surface. For the flat dehydrated (100) surface, the 5-fold-coordinated Al, 3-fold-coordinated O, and 4-fold-coordinated O are exposed. Although both surfaces contain coordinately unsaturated Al and O sites, they differ in their geometry and electron structure because of the degree of surface unevenness and unsaturation. Thus, there are both similarities and differences in their adsorption performance toward Run (n = 1–4) clusters. | ||
| Fig. 2 Top and side views of the dehydrated (110), dehydrated (100), hydrated (110), and hydrated (100) surfaces. Red, pink, and white balls represent O, Al, and H atoms, respectively. | ||
Water, which is usually present on γ-Al2O3 surfaces during preparation, can influence the characteristics of surface sites and the material's adsorption ability or reactivity.10,28,40–42 Thus, in this study, we investigated the interaction of Run (n = 1–4) clusters with hydrated γ-Al2O3 (110) and (100) surfaces. For the hydrated (110) and (100) surfaces, a single H2O molecule was adsorbed on the surfaces to elucidate the partially hydroxylated structure. After testing hydroxylation process (Table S2, ESI†), a hydroxyl-covered (110) surface (θ = 2.94 OH per nm2) is obtained which is identical to the model in the works of Digne et al.31 and Ge et al.43 Similarly, we obtained a (100) surface with θ = 2.12 OH per nm2. These configurations are shown in Fig. 2 H(110) and H(100). The energy barriers for this dissociation pathway are shown in Fig. S4 and S5.† On the hydrated (110) surface, the dissociated H2O molecule interacts with the surface by forming an Ow–Al3c bond (1.71 Å) and a Ha–O3c3 bond (1.03 Å). On the hydrated (100) surface, the favorable configuration consists of Ha adsorbing on the Ob (0.98 Å) and OwHb bonding to Al5c2 (1.76 Å).
3.3 Run (n = 1–4) cluster adsorption on γ-Al2O3 surfaces
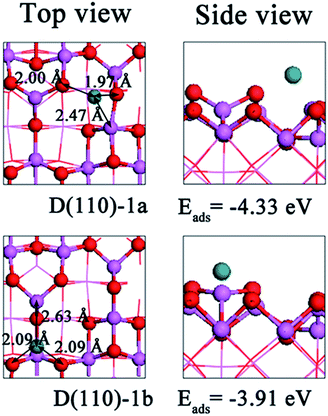
In this section, we illustrate the surface-sensitive interactions between Run (n = 1–4) clusters and the support. Run clusters can interact with the γ-Al2O3 surface through different adsorption sites in different ways. We analyzed various adsorption configurations of Run (n = 1–4) clusters (shown in ESI†) and obtained the corresponding energetic and structural parameters. Here, we only consider the favorable structures.| Configuration | Eads | Eint (eV) | Edef,Run (eV) | Edef,surface (eV) | Ebind(Run/Al2O3) (eV) | Enuc (eV) | d(Ru–Ru) (Å) |
|---|---|---|---|---|---|---|---|
| D(110)-1a | −4.33 | −5.51 | — | 1.18 | — | — | — |
| D(110)-1b | −3.91 | −5.81 | — | 1.90 | — | — | — |
| D(110)-2a | −3.76 | −5.76 | 0.40 | 1.60 | −5.69 | −2.73 | 2.12 |
| D(110)-2b | −2.82 | −4.52 | 0.08 | 1.62 | −5.22 | −1.79 | 2.01 |
| D(110)-3 | −4.73 | −7.05 | 0.15 | 2.17 | −5.78 | −1.63 | 2.38 |
| D(110)-4 | −5.18 | −7.60 | 0.42 | 2.00 | −6.29 | −2.76 | 2.44 |
To further illustrate the modes of cluster adsorption on the substrate, we performed population (Table 3) and electron density difference (Fig. S10†) analyses. Table 3 shows that the Ru atom has a charge of 0.25|e| in the D(110)-1a state, indicating that the charge migrated from the cluster to the surface. Moreover, the deposition of Ru atoms increases the charge density on Al4c atoms and decreases the charge density on O2c1 and O2c2 atoms. Therefore, the observed interactions between Ru atoms and the surface are similar to back-donation interactions,15 where the Ru atom accepts the charge from the O2c1 and O2c2 sites and then donates it to the Al4c site. The net effect of adsorption is that the Ru atom acts as a conduit and thus promotes the charge transfer from the O sites to the Al site. This phenomenon is qualitatively described by determining the charge density difference (Fig. S10†, D(110)-1a), which indicates that the Ru atom is electronically depleted and balanced by the accumulated charge density on the Ru–Al bond and reduced charge density on the Ru–O bonds. The same situation is observed for the D(110)-1b configuration, where Ru promotes the charge transfer from the O3c3 and O3c2 sites to the Al3c site.
| Mulliken charges, |e| | Run | O2c1 | O2c2 | O3c1 | O3c2 | O3c3 | Al3c | Al4c |
|---|---|---|---|---|---|---|---|---|
| D(110) | −1.17 | −1.16 | −1.12 | −1.13 | −1.13 | 1.78 | 1.79 | |
| D(110)-1a | 0.25 | −0.97 | −1.02 | −1.11 | −1.13 | −1.13 | 1.74 | 1.40 |
| D(110)-1b | 0.17 | −1.16 | −1.14 | −1.11 | −0.94 | −0.94 | 1.38 | 1.77 |
| D(110)-2a | 0.32 | −1.07 | −1.02 | −1.10 | −1.01 | −1.10 | 1.39 | 1.50 |
| D(110)-2b | 0.26 | −1.16 | −1.14 | −1.11 | −0.99 | −0.99 | 1.39 | 1.78 |
| D(110)-3 | 0.37 | −1.13 | −1.02 | −0.97 | −0.98 | −1.08 | 1.37 | 1.42 |
| D(110)-4 | 0.48 | −1.02 | −1.04 | −1.00 | −1.01 | −1.10 | 1.37 | 1.40 |
The above discussion indicates that the metal–substrate interactions lead to the formation of new bonds (Ru–O and Ru–Al) at the cluster–support interface and provide the impetus for electron movement from the O sites to the Al site. Among the other adsorption sites, Ru atoms prefer to adsorb at the O2c2⋯O2c1⋯Al4c site to avoid the excessive energy resulting from surface deformation.
Next, Ru2 adsorption on the dehydrated (110) surface was studied. We achieved two stable configurations, which are indicated as D(110)-2a and D(110)-2b. The geometries of the stable configurations are presented in Fig. 4, and the key energies and structural parameters are shown in Table 2. Fig. 4 shows that the adsorption sites of the Ru2 cluster are similar to those of the isolated Ru atom and involve both metal and O atoms. Between the two stable configurations, D(110)-2a is the most stable structure, with an adsorption energy of −3.76 eV, resulting in the formation of Ru1–O2c1, Ru1–O2c2, Ru1–Al4c, Ru2–Al3c, and Ru2–O3c2 bonds. Unlike in the D(110)-2a configuration, the Ru2 cluster interacts with the surface in the D(110)-2b configuration by forming Ru–Al3c (2.55 Å), Ru–O3c2 (2.15 Å), and Ru–O3c3 (2.15 Å) bond and has an adsorption energy of −2.82 eV. The larger number of bonds formed in the D(110)-2a configuration is related to its lower interaction energy (−5.76 eV) relative to the D(110)-2b configuration (−4.52 eV). Because both configurations involve the Al3c site, the surfaces experience similar deformation energies. After geometry optimization, the Ru–Ru distance is 2.01 Å in D(110)-2b configuration, which is shorter than that in the D(110)-2a configuration (2.12 Å) but longer than that of the isolated Ru2 cluster (1.96 Å). Thus, the deformation energy of the Ru2 cluster in D(110)-2b is lower than that in the D(110)-2a configuration. Therefore, the D(110)-2a configuration is more stable than D(110)-2b in terms of the adsorption energy. This difference is especially large in terms of the interaction energy but is balanced by the small difference in the deformation energy costs. Table 2 shows that although Ru2 clusters interact with the surface more strongly than Ru atoms, its higher deformation energy largely neutralize the interaction energy, thereby increasing its adsorption energy. Therefore, the adsorption configuration of an Ru atom in D(110)-1a is more stable than that of an Ru2 cluster in D(110)-2a.
The strong interactions in the D(110)-2a and D(110)-2b configurations are attributed to electron transfer between cluster and surface, where the Ru2 cluster acts as a reservoir, receiving and donating electrons. Table 3 shows that in D(110)-2a and D(110)-2b, the electron density of the O2c1, O2c2, O3c2, and O3c3 sites bonding to the Ru2 cluster is depleted, while that of Al3c and Al4c is enriched. Fig. S10† shows that for D(110)-2a and D(110)-2b, the Ru2 cluster loses its electron, thereby increasing the Ru–Al bond density and decreasing the Ru–O bond density. These findings constitute a qualitative basis for understanding metal–substrate interactions.
Finally, we studied the adsorption of Ru3 and Ru4 clusters on the dehydrated (110) surface. Fig. 5 shows that in the most stable configuration (D(110)-3), the Ru3 cluster is slanted on the surface, which is consistent with previous results obtained for Cu3 clusters.26 In this configuration, the three Ru atoms binds to the surface through Ru1–O2c2, Ru1–Al3c, Ru2–Al3c, Ru2–O3c2, Ru3–O3c1, and Ru3–Al4c bonds. The corresponding bond lengths are 2.09 Å, 2.66 Å, 2.65 Å, 2.09 Å, 2.16 Å, and 2.37 Å, respectively. The average Ru–Ru bond length is 0.10 Å longer than that of isolated Ru3 clusters, resulting in deformation energy of 0.15 eV in the cluster. The increased number of bonds formed in D(110)-3 relative to D(110)-2a yields a stronger interaction between Ru3 and the substrate, with an interaction energy of −7.05 eV. After the Ru3 cluster is deposited, the original appearance of the surface changes, increasing in energy by 2.17 eV, more than D(110)-2a. Additionally, the interaction energy makes a major contribution to the adsorption energy, and as a result, the adsorption energy of the D(110)-3 configuration is 0.97 eV less stable than that of the D(110)-2a configuration. As listed in Table 3, the charge density of the Al atoms (Al3c and Al4c) is more positive and that of the O atoms (O2c2, O3c1, and O3c2) is more negative than in the isolated surface. The total charge of the Ru3 cluster is 0.37|e|, which exceeds that of the Ru2 cluster. The charge density difference shown in Fig. S11† for D(110)-3 suggests that the Ru3 cluster accepts the electron from the bound O atom and then donates it to the Al atoms.
 | ||
| Fig. 5 Top and side views of the most energetically favorable geometries of Ru3 and Ru4 clusters on the dehydrated γ-Al2O3 (110) surface. | ||
For the Ru4 cluster, the most stable structure is shown in Fig. 5 (D(110)-4), in which three Ru atoms interact directly with the surface, forming four Ru–O bonds and two Ru–Al bonds. The adsorption energy of the Ru4 cluster is −5.18 eV, which lower than that of the Ru3 cluster. The small difference in the adsorption energy between the Ru4 and Ru3 clusters is the result of its lower interaction energy and similar deformation energy, as shown in Table 3. These can be attributed to the fourth Ru atom that binds via the Ru–Ru interaction, while the third Ru atom binds via the Ru–support and Ru–Ru interactions. As for the Ru4 cluster deposited on the substrate, the charge density is observed to increase on the Al sites (Al3c and Al4c) and decrease on the O sites (O2c1, O2c2, O3c1, and O3c2). Additionally, the characteristic of Ru4 cluster changes to electropositive (0.48|e|) since adsorption on the surface. Thus, the Ru4 cluster enhances the intensity of the electronic exchange between the O and Al sites. The charge density difference plots shown in Fig. S11† (D(110)-4) reveals increased charge density on the newly formed Ru–Al bond and decreased charge density on the Ru–O bond.
To evaluate the relative stability between different sizes, we also calculated the binding energies and nucleation energies of Run (n = 2–4) clusters as shown in Table 2. It is observed that the support stabilizes the Run clusters by decreasing the binding energies of supported configurations lower than that corresponding gas phase. Additionally, the nucleation process of Run (n = 2–4) clusters is exothermic and thermodynamically favorable suggesting the critical cluster size is 2. This result is in accord with the previous studies on the growth of Rh,27 Cu,38 Ni28 and Pd.44
As stated above, the interaction energy makes a dominant contribution to the adsorption energy, while the deformation energy exerts an opposite effect. The adsorption energies are balanced by the decrease interaction energies and increase deformation energies. For the most stable configurations (D(110)-1a, D(110)-2a, D(110)-3, and D(110)-4), as the cluster size (n = 1–4) increases, the interaction energy decreases. However, this decreasing trend in the adsorption energy only occurs for clusters from Ru2 to Ru4. This is because every cluster adsorption configuration involves Al3c and cluster deformation, except D(110)-1a. Therefore, the higher deformation energy for the surface and the cluster in D(110)-2a increases the adsorption energy relative to that of D(110)-1a. Additionally, the Ru–Ru bond length is extended compared to that of the isolated cluster but is always shorter than that of the bulk. The stable structures requires the cooperative effect of O and metal sites so that the Run (n = 1–4) clusters receive electrons from oxygen sites and donate them to metal sites. Furthermore, the electron transfer increases as the interaction energy decreases (except in the D(110)-1b configuration); the D(110)-2b configuration exhibits the least electron transfer. Therefore, the presence of Run is responsible for increasing the transfer of electrons from O sites to Al sites.
 | ||
| Fig. 6 Top and side views of the most energetically favorable geometries of Run (n = 1–4) clusters on the dehydrated (100) surface. | ||
| Mulliken charges, |e| | Run | Oa | Ob | Oc | Od | Oe | Al5c1 | Al5c2 | Al5c3 |
|---|---|---|---|---|---|---|---|---|---|
| D(100) | — | −1.17 | −1.11 | −1.11 | −1.07 | −1.10 | 1.70 | 1.65 | 1.66 |
| D(100)-1 | 0.07 | −1.15 | −1.10 | −0.97 | −0.94 | −1.10 | 1.67 | 1.32 | 1.63 |
| D(100)-2 | 0.19 | −1.14 | −1.03 | −0.99 | −0.95 | −1.14 | 1.70 | 1.46 | 1.68 |
| D(100)-3 | 0.04 | −1.14 | −0.96 | −0.99 | −0.93 | −1.12 | 1.50 | 1.36 | 1.66 |
| D(100)-4 | 0.18 | −1.15 | −0.98 | −1.01 | −1.01 | −1.04 | 1.52 | 1.36 | 1.48 |
In terms of the adsorption energy (Table 5), the D(100)-1 configuration is the most stable structure among the observed configurations and is exothermic by 4.62 eV. The reason for this stability is that its relatively small deformation energy (1.62 eV) only neutralizes part of the relatively interaction energy (−6.24 eV). In the adsorption of Ru2 clusters, the Ru–Ru bond length is elongated to 2.14 Å with deformation energy of 0.53 eV. In addition, the adsorption of Ru2 dimers induces a large rearrangement of the surface in which the Al5c2 stretched out of the lattice, negatively affecting adsorption. Additionally, the Ru–Al2O3 interaction is 1.05 eV weaker than that in D(100)-1. These effects contribute to the higher adsorption energy of the D(110)-2 configuration compares with the D(100)-1 configuration. For Ru3 cluster adsorption, the adsorption energy is −3.66 eV, more exothermic than the Ru2 particle. This is because of its lower interaction energy and lower deformation energy cost. As for the Ru3 cluster, the most favorable configuration for Ru4 clusters is slant on the surface with two Ru atoms bound to the surface; this configuration is less exothermic (0.35 eV) than the D(100)-3 configuration. Although the deformation introduced by Ru4 cluster adsorption is 0.37 eV smaller than that of the Ru3 cluster, the interaction energy is 0.72 eV higher than in the D(100)-3 configuration, resulting in an adsorption energy (−3.31 eV) higher than that of the Ru3 cluster but lower than that of the Ru2 cluster. Additionally, as the cluster sizes increase, the binding energies decrease. Be similar to dehydrated (110), the critical size for cluster is 2. However, the nucleation reaction on dehydrated (110) surface is more favored than on (100) surface.
| Configuration | Eads (eV) | Eint (eV) | Edef,Run (eV) | Edef,surface (eV) | Ebind(Run/Al2O3) (eV) | Enuc (eV) | d(Ru–Ru) (Å) |
|---|---|---|---|---|---|---|---|
| D(100)-1 | −4.62 | −6.24 | — | 1.62 | — | — | — |
| D(100)-2 | −2.18 | −5.19 | 0.53 | 2.48 | −4.90 | −0.59 | 2.14 |
| D(100)-3 | −3.66 | −5.97 | 0.16 | 2.15 | −5.42 | −1.86 | 2.37 |
| D(100)-4 | −3.31 | −5.25 | 0.17 | 1.77 | −5.82 | −2.41 | 2.43 |
The above results indicate that the adsorption energies are highly dependent on and increase with the interaction energies. Because of its relatively strong interactions and relatively small deformation, the D(100)-1 configuration is the most stable. Comparing the adsorption on the D(110) and D(100) surfaces reveals that the interaction energies and adsorption energies for Run (n = 2–4) clusters on the D(110) surface are smaller than those on the D(100) surface, suggesting that the stronger metal–support interactions should occur on the D(110) surface. However, the opposite is observed for Ru atom adsorption. The population analysis and charge density difference analysis of the D(100) surface suggests that the interactions between Ru atoms and γ-Al2O3 in these configurations is similar a donation and back-donation interaction where the Ru cluster acts as a conduit, moving charge from O to Al sites. However, consistent with adsorption on the D(110) surface, the electron variation in the involved sites is relatively small. The origin of this phenomenon will be analyzed in detail in the Section 3.4.
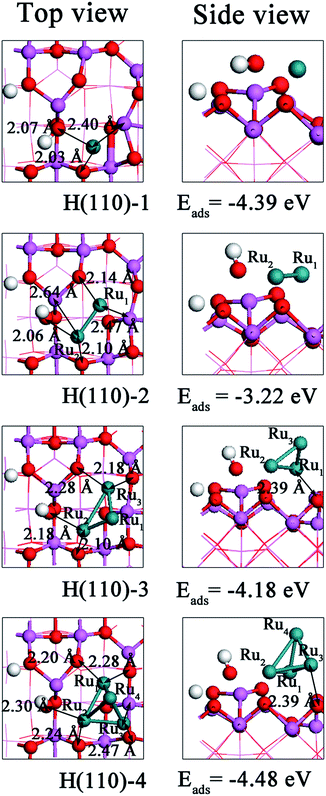 | ||
| Fig. 7 Top and side views of the most energetically favorable geometries of Run (n = 1–4) clusters on the hydrated (110) surface. | ||
| Mulliken charges, |e| | Run | O2c1 | O2c2 | O3c1 | O3c2 | Ow | Al3c | Al4c |
|---|---|---|---|---|---|---|---|---|
| H(110) | −1.14 | −1.20 | −1.12 | −1.13 | −1.13 | 1.85 | 1.80 | |
| H(110)-1 | 0.22 | −1.13 | −1.18 | −1.06 | −0.96 | −0.95 | 1.80 | 1.37 |
| H(110)-2 | 0.35 | −1.06 | −1.05 | −1.10 | −0.99 | −0.93 | 1.69 | 1.36 |
| H(110)-3 | 0.33 | −0.99 | −1.06 | −1.08 | −1.00 | −0.95 | 1.78 | 1.27 |
| H(110)-4 | 0.36 | −1.03 | −1.06 | −1.02 | −1.01 | −0.96 | 1.69 | 1.30 |
In terms of the adsorption energy (Table 7), the H(110)-4 configuration is the most stable structure among those shown in Fig. 7. Although the interaction energy in H(110)-4 is 0.24 eV higher than that in H(110)-1, its deformation energy is 0.33 eV lower. Thus, the deformation energy only neutralizes a small portion of the interaction energy, resulting in adsorption energy of −4.48 eV. For Ru atom adsorption, the Al4c atom is distorted from its original location and is endothermic by 1.89 eV. Additionally, the interaction energy of H(110)-1 is the lowest. Thus, the H(110)-1 configuration has the second highest adsorption energy, behind H(110)-4. For the H(110)-2 configuration, although its deformation energy is lower than that of H(110)-1, there is a large difference in the interaction energies between these two configurations, and as a result, the adsorption energy of H(110)-2 is 1.17 eV less exothermic than that of H(110)-1. For Ru3 cluster adsorption, the lower interaction energy in conjunction with the lower deformation energy lowers the adsorption energy below that of Ru2 cluster adsorption. Moreover, the binding and nucleation energy for Ru4 cluster on hydrated (110) surface is the lowest among others.
| Configuration | Eads (eV) | Eint (eV) | Edef,Run (eV) | Edef,surface (eV) | Ebind(Run/Al2O3) (eV) | Enuc (eV) | d(Ru–Ru) (Å) |
|---|---|---|---|---|---|---|---|
| H(110)-1 | −4.39 | −6.28 | — | 1.89 | — | — | — |
| H(110)-2 | −3.22 | −4.94 | 0.01 | 1.71 | −5.42 | −5.38 | 2.14 |
| H(110)-3 | −4.18 | −5.83 | 0.16 | 1.49 | −5.58 | −5.62 | 2.37 |
| H(110)-4 | −4.48 | −6.04 | 0.20 | 1.36 | −6.11 | −5.92 | 2.43 |
The above results indicates that the adsorption energy decreases as the cluster size increases, except for Ru atom adsorption, which is consistent with the trend observed for the interaction energy.
However, the deformation energy for Run (n = 1–4) cluster adsorption configurations decreases as the cluster size increases. Comparing adsorption on the hydrated (110) and dehydrated (110) surfaces reveals that the adsorption energies for Run (n = 1–4) clusters on the dehydrated (110) surface converges to within 0.4–1.42 eV, whereas the adsorption energies for Run (n = 1–4) clusters on the hydrated (110) surface converges to within 0.09–1.26 eV. This finding suggests that the energy variations decrease because of the presence of surface hydroxyl groups. Additionally, the interaction energies of Run (n = 2–4) clusters on the H(110) surface are higher than those on the D(110) surface, indicating that the presence of the hydroxyl groups on the (110) surface weakens the interactions. The opposite is observed for Ru atom adsorption. Because the Ow participated in the adsorption on the hydrated (110) surface instead of Al3c, the increased interaction energy could not be “neutralizes” by decreased deformation energy. As a result, the adsorption of Run (n = 2–4) clusters on the hydrated (110) surface is more stable than that on the dehydrated (110) surface. In contrast, the Ru atom adsorbs more stably on the hydrated (110) surface than on the dehydrated (100) surface. The interaction mechanism in which the Run (n = 1–4) cluster acts as a medium to accelerate the transfer of electrons from the O sites to the Al sites is the same for both the hydrated and the dehydrated (110) surface. However, when the variation in the Mulliken charge is compared, we observe less electron movement on the hydrated surface. It is observed that the hydroxyls reduce the stability of Run (n = 2–4) clusters on (110) surface. Therefore, the introduction of hydroxyl groups also introduces some changes.
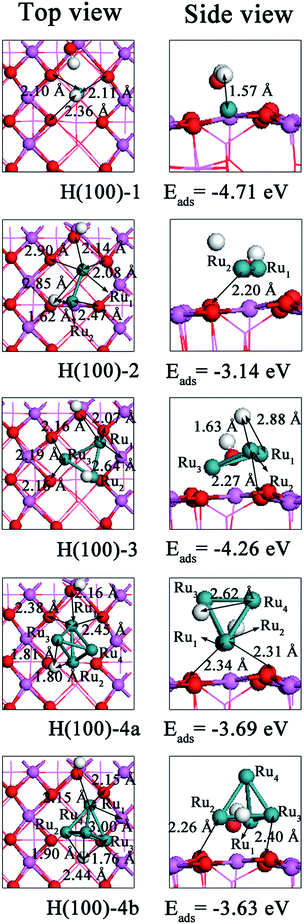 | ||
| Fig. 8 Top and side views of energetically favorable geometries of Run (n = 1–4) clusters on the hydrated (100) surface. | ||
| Mulliken charges, |e| | Run | Oa | Ob | Oc | Od | Ow | Ha | Al5c4 |
|---|---|---|---|---|---|---|---|---|
| H(100) | −1.17 | −1.03 | −1.12 | −1.10 | −1.11 | 0.48 | 1.68 | |
| H(100)-1 | 0.53 | −1.06 | −1.02 | −1.01 | −0.98 | −1.04 | −0.16 | 1.66 |
| H(100)-2 | 0.60 | −1.09 | −0.99 | −1.02 | −1.07 | −0.95 | −0.20 | 1.68 |
| H(100)-3 | 0.61 | −1.05 | −0.99 | −0.96 | −0.99 | −0.94 | −0.20 | 1.44 |
| H(100)-4a | 0.51 | −1.05 | −1.00 | −1.04 | −0.99 | −0.95 | −0.18 | 1.67 |
| H(100)-4b | 0.54 | −1.05 | −1.00 | −0.99 | −1.07 | −0.95 | −0.14 | 1.52 |
In terms of the adsorption energy (Table 9), H(100)-1 is the most stable structure among those shown in Fig. 8 and is exothermic by 4.71 eV. The underlying reason for this finding is that the cluster and surface suffer the smallest deformations possible and that the interaction energy (−10.36 eV) is the second lowest (second to that of H(100)-3: −11.09 eV). For Ru2 cluster adsorption, the interaction energy is 0.94 eV higher than that of the H(100)-1 configuration. In addition, the surface and cluster both experience greater deformation than in the H(100)-1 configuration, which contributes 6.28 eV to the interaction energy. Therefore, the adsorption energy of the H(100)-2 configuration is higher than that involved in Ru atom adsorption. For Ru3 cluster adsorption, the adsorption energy is −4.26 eV, the second lowest value. This value could be related to the large gap in interaction energy and the cluster's larger deformation energy and surface cost. Although the deformation energy for H(100)-4a is 0.57 eV lower than that of H(100)-3, it could not balance the 1.14 eV gap in the interaction energy. Thus, the adsorption energy of the H(100)-4a is 0.57 eV higher than that associated with Ru3 cluster adsorption. Another stable configuration for Ru4 cluster adsorption is the H(100)-4b configuration, in which the metal–support interaction energy is only −9.58 eV. This adsorption configuration needs to trap 5.95 eV to satisfy its deformation cost. Consequently, the deposition of Ru clusters causes the H(100)-4b configuration to be exothermic by 3.63 eV, 0.06 eV less than H(100)-4b. Additionally, the binding energies shown in Table 9 indicate the Ru4 cluster is the most stable configurations than others. And it is thermodynamically favorable to grow Run (n = 2–4) clusters on hydrated (110) surface.
| Configuration | Eads (eV) | Eint (eV) | Edef,Run (eV) | Edef,surface (eV) | Ebind(Run/Al2O3) (eV) | Enuc (eV) | d(Ru–Ru) (Å) |
|---|---|---|---|---|---|---|---|
| H(100)-1 | −4.71 | −10.36 | — | 5.65 | — | — | — |
| H(100)-2 | −3.14 | −9.42 | 0.29 | 5.99 | −5.38 | 1.34 | 2.08 |
| H(100)-3 | −4.26 | −11.09 | 0.23 | 6.60 | −5.62 | −1.40 | 2.33 |
| H(100)-4a | −3.69 | −9.95 | 0.18 | 6.08 | −5.92 | −2.08 | 2.41 |
| H(100)-4b | −3.63 | −9.58 | 0.24 | 5.71 | −5.90 | −2.03 | 2.40 |
The above results indicate the adsorption energy is a combination of the interaction energy and the deformation energy. As shown in Table 8, we rank the adsorption energies of Run (n = 1–4) clusters on the hydrated (100) surface as follows: H(100)-2 > H(100)-4b > H(100)-4a > H(100)-3 > H(100)-1. This suggests that the H(100)-1 configuration is the most stable structure. Comparing the data in Tables 4 and 8, the adsorption energies for Run (n = 1–4) clusters on the dehydrated (100) surface converge to within 0.35–2.44 eV, whereas the adsorption energies for Run (n = 1–4) clusters on the hydrated (100) surface converge to within 0.06–1.57 eV, indicating that introducing surface hydroxyl groups lessen the energy variation. Additionally, we observe that the adsorption energy and interaction energy of the hydrated (100) surface are more negative than those of the dehydrated (100) surface, suggesting that introducing hydroxyl groups to the (100) surface strengthen the interactions. We also find that in the adsorption configurations, the Ha tends to stay away from Ob but approach the Run (n = 1–4) clusters. As a result, the hydrated surface undergoes greater deformation than the dehydrated (100) surface. In general, the introduction of the surface hydroxyl groups not only influences the adsorption energy but also affects the interaction mechanism. On the hydrated (100) surface, the interaction mechanism in which the Run (n = 1–4) cluster acted as a medium to accelerate the electron transfer from the O sites to Ha (Al4c) sites is different from that on the dehydrated surface. In addition, comparing the Mulliken charges, we find more electron migration occurs on the hydrated surface, which responsible for the red-shift of CO absorbance.33 This result is in consistent with the previous study on Ru/silica and Ru/zeolite catalysts.45 Clearly, given the information in provided in the section “Run (n = 1–4) clusters adsorption on dehydrated γ-Al2O3 (100) surface”, the surface hydroxyl groups exert different effects on the adsorption behavior of Run (n = 1–4) clusters on the hydrated (110) and (100) surfaces. Therefore, the population analysis and charge density difference analysis could not explain the differences. As a result, studies conducted from additional perspectives are needed. Moreover, the hydroxyls enhance the stability of clusters on (100) surface.
3.4 Site-dependent Lewis acidity and basicity of surface: PDOS analysis
Understanding cluster–support interactions will contribute to the design and fabrication of heterogeneous catalysts with desirable stability and activity properties. The above sections discussed in detail the adsorption configuration from the perspective of geometric structure and corresponding energies, and the population and charge density difference analyses provided information about metal–support interactions. However, the primary origin of the different adsorption behaviors remains to be determined.It is well known that the atoms' intrinsic Lewis acid/basicity is associated with charge transfer. Therefore, a detailed investigation of the Lewis acid/basicity of that atoms that participate in the adsorption configurations was performed. Prior works have investigated the Lewis acidity of surface alumina sites in terms of the energy of unoccupied bands and have reported that lower energy levels correlate with alumina sites with stronger Lewis acidity, thereby increasing the atom's adsorption stability. Surface O anions usually act as “basic” centers that provide charges to clusters. The Lewis basicity of O sites can be evaluated in terms of the energy of the highest occupied bands: higher energies indicate stronger donor abilities.46,47 Therefore, the projected electronic density of states (PDOS) was used to characterize the intrinsic reactivity of surface alumina and O sites. Because the Lewis acid acts as an electron acceptor, we only considered the PDOS of the 3s3p orbital of surface Al (the lowest unoccupied bands in alumina), and used the band center to describe its Lewis acidity as follows:
 | (8) |
The Lewis basicity of O sites can be defined similarly by considering the PDOS of the 2s2p orbital (the highest occupied bands). The band center can be described as follows:
 | (9) |
For Al sites, a lower value of ε indicates stronger acidity and, thus, a stronger ability to accept electrons from donors. Similarly, because the O anion 2s2p orbital has the highest energy, it is also the most basic. Thus, we determine the ε values for Al sites and O sites on different surfaces, as listed in Table 10 and 11. The calculated PDOS are presented in the ESI.†
| Dehydrated (110) | Al3c | Al4c | |||
| ε | 5.37 | 5.64 | |||
| Hydrated (110) | Al3c | Al4c | Ha | ||
| ε | 6.61 | 5.26 | 6.87 | ||
| Dehydrated (100) | Al5c1 | Al5c2 | Al5c3 | Al5c4 | |
| ε | 5.87 | 5.34 | 5.82 | 5.88 | |
| Hydrated (100) | Al5c1 | Al5c2 | Al5c3 | Al5c4 | Ha |
| ε | 6.03 | 6.66 | 5.99 | 5.74 | 5.17 |
| Dehydrated (110) | O2c1 | O2c2 | O3c1 | O3c2 | O3c3 | |
| ε | −6.71 | −7.18 | −7.62 | −7.90 | −7.90 | |
| Hydrated (110) | O2c1 | O2c2 | O3c1 | O3c2 | O3c3 | Ow |
| ε | −7.86 | −7.02 | −7.75 | −7.90 | −8.04 | −7.57 |
| Dehydrated (100) | Oa | Ob | Oc | Od | Oe | |
| ε | −7.81 | −7.29 | −7.49 | −7.24 | −7.49 | |
| Hydrated (100) | Oa | Ob | Oc | Od | Oe | Ow |
| ε | −7.78 | −9.39 | −6.86 | −7.04 | −7.56 | −6.45 |
Our geometry and population analysis predict that the presence of electropositive Run clusters was a result of accumulating charge density on the Ru–Al bond and depleting on the Ru–O bond. It is therefore qualitative reasonable to say that two Lewis acid–base pairs are on the system. In other words, a Run cluster is an acid in Ru–O pair while be a base in Ru–Al bond.48 The above data reveals that, as expected, the low-coordinated sites are more acidic than the highly coordinated sites on the dehydrated surface. Nevertheless, some exceptions are observed: the Al5c2 site on the dehydrated (100) surface is more acidic than the Al4c site on the dehydrated (110) surface, indicating that the coordinate number is not the only factor that influences the acidity. By analyzing and comparing the sites' Lewis acidities, the dehydrated (110) surface is more acidic than the (100) surface; namely, the aluminum sites on the (110) surface are better able to receive electrons from Ru atoms. Furthermore, the information in Table 11 allows the following ranking to be constructed with regard to basicity on the dehydrated surface: O2c1 > O2c2 > Od > Ob > Oc = Oe > O3c1 > O3c2; only the sites involved in the most stable configuration are analyzed. After comparing the sites' average basicity values, a subtle difference between the two surfaces becomes evident. Although the average basicity of (110) is roughly equal to that of (100) surface, the absence of electron induced by Ru–Al bond turns the Run cluster into a strong Lewis acid, strengthening the Ru–O bond.49 Thus, the stronger acceptor and nearly equal donor sites on the dehydrated (110) surface drive more electron transfer between clusters and the surface, indicating that the adsorption of Run (n = 2–4) clusters on the dehydrated (110) surface is stronger than that of Run (n = 2–4) clusters on the dehydrated (100) surface. Considering the work of Akane et al.,14 if we want to minimize the cluster–support interactions for ammonia synthesis, we should expose the (100) surface more than others. The adsorption of Ru atoms is, of course, the exception to the rule. Comparing the ε values of O2c1, O2c2, Al4c, Oc, Od, and Al5c2 sites involved in bonds in the D(110)-1a and D(100)-1 configurations reveals that O2c1 and O2c2 are more basic than Oc and Od, but Al4c is less acidic than Al5c2. As a weak acid, Ru atom may be able to accept few electrons from O site; the existence of a stronger base (O2c1 and O2c2) makes subtle difference when compared to Oc and Od in Ru–O bond.49 Conversely, a more acid (Al5c2 site) would increase the Ru–Al bond strength. Therefore, the relatively high level of electron movement on the dehydrated (110) surface relative to the dehydrated (100) surface represents the combine effects of higher donor ability and lower acceptor ability; this trend is opposite to that observed for the adsorption ability.
As shown in Table 10, the Al3c ε value increases when hydroxyl groups adsorbed on the Al3c site whereas that of Al4c decreases. A similar situation appears to hold for the basicity of the O sites: the basicity of the O2c1, O3c1, and O3c3 sites decreases, while that of the O2c2 site increases. By comparing the variation in the ε value, we determine that the net electron acceptor ability for Al sites and donor ability for oxygen sites decreases because of the introduction of surface hydroxyl groups. Consequently, Run (n = 2–4) clusters accepted less electrons from O sites and thereby was less basic in Ru–Al bond when compared to dry surface. Thus, these groups increases the adsorption energy and interaction energy of the adsorption of Run (n = 2–4) clusters on the hydrated (110) surface. The opposite is observed for the adsorption of Ru atoms. Comparing the ε values of the O3c2, Ow, Al4c (hydrated), O2c1, O2c2, and Al4c (dehydrated) sites involved in bonding in the H(110)-1 and D(110)-1a configurations reveals that O3c2 and Ow sites are less basic than O2c1 and O2c2, whereas Al4c (hydrated) is more acidic than Al4c (dehydrated), which plays a dominant role. Therefore, more electron movement occurs when Ru atom adsorption took place on the hydrated (110) surface than on the dehydrated (110) surface, decreasing the adsorption energy and interaction energy.
Based on the above discussion of energies, the surface hydroxyl exerts different effects on the adsorption behavior on the (100) surface than on the (110) surface. Thus, the introduction of hydroxyl groups must have changed the surface in some way. The ε values shown in Table 6 indicates that the surface hydroxyl groups decrease the Lewis acidity of the hydrated (100) surface sites, except for the Al5c4 site. However, the Lewis acidity of the Ha involved in bonding is stronger than that of the Al sites, which explains the cluster–substrate interactions based on electron transfer from the Ru atom to the Ha (Al) sites. The acidity of Ha is also stronger than that of the Al sites on the dehydrated (100) surface. It is therefore more electrons were migrated from Run (n = 1–4) clusters to Ha atom than to Al atom on dehydrated (100) surface. Although the Lewis basicities of the Ob and Oe sites decrease, the hydrated (100) surface includes relatively strongly basic Ow, Oa, Oc, and Od sites. Overall, the basicity of the hydrated (100) surface is stronger than that of the dehydrated (100) surface. As a result, more basic O sites together with stronger acid Run clusters increase the stability of Ru–O bonds. Therefore, the stronger adsorption on the hydrated (100) relative to the dehydrated (100) surface represents a combined effect of the increased donor ability and acceptor ability. To support this claim, we calculated relationships between the average (εAl − εO) values of the O and Al atoms involved in bonding and interaction energies (as shown in Fig. S22†). The results clearly show that interaction energies increase with the (εAl − εO) values (i.e., stronger Lewis acid–basicity pair leads to stronger interaction).
It is found that the adsorption performance of a single Ru atom exhibits distinctive stability other than Run (n = 2–4) clusters. This means the supported single-Ru atom catalyst would show characteristic reactivity and selectivity in chemical process; for instance, catalyzing the CO2 into CO instead of CH4.26 Moreover, the remarkable performance of supported single-atom catalysts has attracted extensive attention.50–52 For example, Zhang et al. reported the activity of Ir1/FeOx in water gas shift is 1 order of magnitude higher than its clusterpart.50 Studies for catalysis performance of supported single-Ru atom are in progress.
4. Conclusions
In this work, to investigate the influence of cluster size, surface structure, and hydroxyl groups on adsorption behavior and interactions, DFT calculations were conducted to characterize the adsorption and nucleation of Run (n = 1–4) clusters on γ-Al2O3 (110) and (100) surfaces. The results indicate that the adsorption process is strongly sensitive to the cluster size and the surface microstructure (atomic and electronic structure) and environment. The adsorption energy is the product of coordination between the deformation energy and interaction energy. A single Ru atom prefers to adsorb on the (100) surface in the presence of small deformation, whereas strong metal–support interactions lead to the adsorption of Ru4 clusters on the (110) surface. When the surface is dehydrated, the adsorption energy and interaction energy of Run (n = 2–4) clusters adsorbed on the (110) surface are smaller relative to those of the (100) surface, whereas the opposite is observed for single Ru atom adsorption. The population and charge density difference analyses shows that the adsorption is stabilized by the O (donor)–Ru–Al (acceptor) charge movement effect. The stronger acceptor and nearly equal donor sites on the (110) surface drive increased electron movement, making the (110) surface relatively favorable for Run (n = 2–4) cluster adsorption.The introduction of hydroxyl groups decrease the acceptor ability of the Al sites and the donor ability of the O sites in the (110) surface, thereby reducing the driving force for electron transfer and lowering the adsorption stability of Run (n = 2–4) clusters on the hydrated (110) surface. However, single Ru atoms preferentially adsorbs on the hydrated (110) surface over the dehydrated (110) surface because of the increased acidity of the Al4c site. Conversely, the Ha acts as an active site and increases the stability of Run (n = 1–4) cluster adsorption on the (100) surface. Specifically, the overall basicity of the O sites increase because of the introduction of surface hydroxyl groups. Thus, the electron transfer from the O sites to the Ha site proceeds through Run clusters acting as a medium. In summary, the present results demonstrates that the adsorption process of Run (n = 1–4) clusters is surface dependent.
Although the nucleation of Run (n = 2–4) cluster on all surfaces is thermodynamically favorable, the process on γ-Al2O3 support is less stable than in isolated state. The hydroxyls effect on the nucleation process is varied with the exposed surface accompanied with facilitate agglomeration for large cluster on the (110) surface while suppress it on the (100) surface. Moreover, it is obviously that the nucleation process for Ru4 cluster has a larger tendency, namely, the three-dimensional particles will be easily transformed from the two-dimensional. This work provides a theoretical foundation for experimental results, contributing to the design and synthesis of effective catalysts.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 21173131) and the Taishan Scholar Project of Shandong Province (China).Notes and references
- L. X. Cui, Y. H. Tang, H. Zhang, L. C. Ouyang Jr, S. Q. Shi, H. Li and L. Q. Chen, First-principles investigation of transition metal atom M (M = Cu, Ag, Au) adsorption on CeO2(110), Phys. Chem. Chem. Phys., 2012, 14, 1923–1933 RSC.
- Z. Y. Duan and G. Henkelman, CO oxidation at the Au/TiO2 boundary: the role of the Au/Ti5c site, ACS Catal., 2015, 1589–1595 CrossRef CAS.
- S. Aranifard, S. C. Ammal and A. Heyden, On the importance of metal-oxide interface sites for the water–gas shift reaction over Pt/CeO2 catalysts, J. Catal., 2014, 309, 314–324 CrossRef CAS.
- Z. F. Qin, J. Ren, M. Q. Miao, Z. Li, J. Y. Lin and K. C. Xie, The catalytic methanation of coke oven gas over Ni–Ce/Al2O3 catalysts prepared by microwave heating: effect of amorphous NiO formation, Appl. Catal., B, 2015, 164, 18–30 CrossRef CAS.
- L. Ran, Z. Qin, Z. Y. Wang, X. Y. Wang and Q. G. Bai, Catalytic decomposition of CH2Cl2 over supported Ru catalysts, Catal. Commun., 2013, 37, 5–8 CrossRef CAS.
- S. Y. Wu, Y. R. Lia, J. J. Ho and H. M. Hsieh, Density functional studies of ethanol dehydrogenation on a 2Rh/γ-Al2O3(110) surfaces, J. Phys. Chem. C, 2009, 113, 16181–16187 CAS.
- Z. Q. Wang, Z. N. Xu, S. Y. Peng, M. J. Zhang, G. Lu, Q. S. Chen, Y. M. Chen and G. C. Guo, High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation, ACS Catal., 2015, 5, 4255–4259 CrossRef CAS.
- D. H. Mei, V. A. Glezakou, V. Lebarbier, L. Kovarik, H. Y. Wan, K. O. Albrecht, M. Gerber, R. Rousseau and R. A. Dagle, Highly active and stable MgAl2O4-supported Rh and Ir catalysts for methane steam reforming: a combined experimental and theoretical study, J. Catal., 2014, 316, 11–23 CrossRef CAS.
- C. M. Li, S. T. Zhang, B. S. Zhang, D. S. Su, S. He, Y. F. Zhao, J. Liu, F. Wang, M. Wei, D. G. Evans and X. Duan, Photohole-oxidation-assisted anchoring of ultra-small Ru clusters onto TiO2 with excellent catalytic activity and stability, J. Mater. Chem. A, 2013, 1, 2461–2467 CAS.
- D. H. Mei, J. H. Kwak, J. Z. Hu, S. J. Cho, J. Szanyi, L. Allard and C. F. Peden, Unique role of anchoring penta-coordinated Al3+ sites in the sintering of γ-Al2O3-supported Pt catalysts, J. Phys. Chem. Lett., 2010, 1, 2688–2691 CrossRef CAS.
- D. F. Mukhamedzyanova, N. K. Ratmanova, D. A. Pichugina and N. E. Kuzmenko, A structural and stability evaluation of Au12 from an isolated cluster to the deposited material, J. Phys. Chem. C, 2012, 116, 11507–11518 CAS.
- H. Deng, Y. B. Yu, F. D. Liu, J. Z. Ma, Y. Zhang and H. He, Nature of Ag species on Ag/γ-Al2O3: a combined experimental and theoretical, ACS Catal., 2014, 4, 2776–2784 CrossRef CAS.
- A. Bruix, J. A. Rodriguez, P. J. Ramírez, S. D. Senanayake, J. Evans, J. B. Park, D. Stacchiola, P. Liu, J. Hrbek and F. Illas, A new type of strong metal–support interaction and the production of H2 through the transformation of water on Pt/CeO2 (111) and Pt/CeOx/TiO2 (110) catalysts, J. Am. Chem. Soc., 2012, 134, 8968–8974 CrossRef CAS PubMed.
- M. Akane, B. Ioan, A. Ken-ichi and N. Yoshio, Preparation of Ru nanoparticles supported on γ-Al2O3 and its novel catalytic activity for ammonia synthesis, J. Catal., 2001, 204, 364–371 CrossRef.
- J. D. Li, E. Croiset and L. Ricardez-Sandoval, Effect of metal–support interface during CH4 and H2 dissociation on Ni/γ-Al2O3: a density functional theory study, J. Phys. Chem. C, 2013, 117, 16907–16920 CAS.
- G. Pacchioni, Electronic interactions and charge transfers of metal atoms and clusters on oxide surface, Phys. Chem. Chem. Phys., 2013, 15, 1737–1757 RSC.
- S. T. Zhang, C. M. Li, H. Yan, M. Wei, D. Evans and X. Duan, Density functional theory study on the metal–support interaction between Ru cluster and anatase TiO2(101) surface, J. Phys. Chem. C, 2014, 118, 3514–3522 CAS.
- Y. G. Wang, Y. Yoon, V. A. Glezakou, J. Li and R. Rousseau, The role of reducible oxide-metal cluster charge transfer in catalytic process: new insights on the catalytic mechanism of CO oxidation on Au/TiO2 from ab initio molecular dynamics, J. Am. Chem. Soc., 2013, 135, 10673–10683 CrossRef CAS PubMed.
- H. Y. Liu, B. T. Teng, M. H. Fan, B. J. wang, Y. L. Zhang and H. G. Harris, CH4 dissociation on the perfect and defective MgO(001) supported Ni4, Fuel, 2014, 123, 285–292 CrossRef CAS.
- C. T. Campbell, Catalyst–support interactions: electronic perturbations, Nat. Chem., 2012, 4, 597–598 CrossRef CAS PubMed.
- K. R. Hahn, A. P. Seitsonen, M. Lannuzzi and J. Hutter, Functionalization of CeO2(111) by deposition of small Ni clusters: effect on CO2 adsorption and O vacancy formation, ChemCatChem, 2015, 7, 625–634 CrossRef CAS.
- J. Carrasco, D. López-Durán, Z. Y. Liu, T. Duchoň, J. Evans, S. D. Senanayake, E. J. Crumlin, V. Matolín, J. A. Rodríguez and M. V. Ganduglia-Pirovano, In situ and theoretical studies for the dissociation of water on an active Ni/CeO2 catalyst: importance of strong metal–support interactions for the cleavage of O–H bonds, Angew. Chem., Int. Ed., 2015, 54, 3917–3921 CrossRef CAS PubMed.
- N. LóPZ and f. Illas, Electronic effects in the activation of supported metal clusters: density functional theory study of H2 dissociation on Cu/SiO2, J. Phys. Chem. B, 1999, 103, 8552–8557 CrossRef.
- M. Mavrikakis, P. Stoltze and J. K. Nørskov, Making gold less noble, Catal. Lett., 2000, 64, 101–106 CrossRef CAS.
- Z. Kowalczyk, K. Stolecki, W. Raróg-Pilecka, E. Miśkiewicz, E. Wilczkowska and Z. Karpinski, Supported ruthenium catalysts for selective methanation of carbon oxides at very low COx/H2 ratios, Appl. Catal., A, 2008, 342, 35–39 CrossRef CAS.
- J. H. Kwak, L. Kovarik and J. Szanyi, CO2 reduction on supported Ru/Al2O3 catalysts: cluster size dependence of product selectivity, ACS Catal., 2013, 3, 2449–2455 CrossRef CAS.
- X. R. Shi and D. Sholl, Nucleation of Rhn (n = 1–5) clusters on γ-Al2O3 surfaces: a density functional theory study, J. Phys. Chem. C, 2012, 116, 10623–10631 CAS.
- Z. X. Liu, Y. H. Wang, J. R. Li and R. G. Zhang, The effect of γ-Al2O3 surface hydroxylation on the stability and nucleation of Ni in Ni/γ-Al2O3 catalyst: a theoretical study, RSC Adv., 2014, 4, 13280–13292 RSC.
- C. H. Hu, C. Chizallet, C. Mager-Maury, M. Corral-Valero, P. Sautet, H. Toulhoat and P. Raybaud, Modulation of catalyst particle structure upon support hydroxylation: ab initio insights into Pd13 and Pt13/γ-Al2O3, J. Catal., 2010, 274, 99–110 CrossRef CAS.
- F. Wang, S. T. Zhang, C. M. Li, J. Liu, S. He, Y. F. Zhao, H. Yan, M. Wei, D. G. Evans and X. Duan, Catalytic behavior of supported Ru nanoparticles on the (101) and (001) facets of anatase TiO2, RSC Adv., 2014, 4, 10834–10840 RSC.
- M. Digne, P. Sautet, P. Raybaud, P. Euzen and H. Toulhoat, Use of DFT to achieve a rational understanding of acid-basic properties of γ-alumina surfaces, J. Catal., 2004, 226, 54–68 CrossRef CAS.
- H. Petitjean, H. Guesmi, H. Lauron-Pernot, G. Costentin, D. Loffreda, P. Sautet and F. Delbecq, How surface hydroxyls enhance MgO reactivity in basic catalysis: the case of methylbutynol conversion, ACS Catal., 2014, 4, 4004–4014 CrossRef CAS.
- A. S. Baird, K. M. Kross, D. Gottschalk, E. A. Hinson, N. Wood and K. A. Layman, Attenuated Total Reflection Fourier Transform Infrared Spectroscopic Investigation of CO Adsorption on Hydrated Ru/Al2O3, J. Phys. Chem. C, 2007, 111, 14207–14214 CAS.
- A. M. Abdel-Mageed, S. Eckle and R. J. Behm, High selectivity of supported Ru catalysts in the selectivity CO methanation-water makes the difference, J. Am. Chem. Soc., 2015, 137, 8672–8675 CrossRef CAS PubMed.
- M. C. Valero, P. Raybaud and P. Sautet, Influence of the hydroxylation of γ-Al2O3 surfaces on the stability and diffusion of single Pd atoms: a DFT study, J. Phys. Chem. B, 2006, 110, 1759–1767 CrossRef CAS PubMed.
- J. H. Kwak, J. Z. Hu, A. Lukaski, D. H. Kim, J. Szanyi and C. H. F. Peden, Role of pentacoordinated Al3+ ions in the high temperature phase transformation of γ-Al2O3, J. Phys. Chem. C, 2008, 112, 9486–9492 CAS.
- J. H. Kwak, J. Z. Hu, D. H. Kim, J. Szanyi and C. F. Peden, Penta-coordinated Al3+ ions as preferential nucleation sites for BaO on γ-Al2O3: an ultra-high-magnetic field 27Al MAS NNR study, J. Catal., 2007, 251, 189–194 CrossRef CAS.
- J. R. Li, R. G. Zhang and B. J. Wang, Influence of the hydroxylation of γ-Al2O3 surfaces on the stability and growth of Cu for Cu/γ-Al2O3 catalyst: a DFT study, Appl. Surf. Sci., 2013, 270, 728–736 CrossRef CAS.
- Y. X. Pan, C. J. Liu, T. S. Wiltowski and Q. F. Ge, CO2 adsorption and activation over γ-Al2O3-supported transition metal dimers: a density functional study, Catal. Today, 2009, 147, 68–76 CrossRef CAS.
- R. G. Zhang, H. Y. Liu, B. J. Wang and L. X. Ling, Insights into the effect of surface hydroxyls on CO2 hydrogenation over Pd/γ-Al2O3 catalyst: a computational study, Appl. Catal., B, 2012, 126, 108–120 CrossRef CAS.
- Y. X. Pan, C. J. Liu and Q. F. Ge, Effect of surface hydroxyls on selective CO2 hydrogenation over Ni4/γ-Al2O3: a density functional theory study, J. Catal., 2010, 272, 227–234 CrossRef CAS.
- R. G. Zhang, B. J. Wang, H. Y. Liu and L. X. Ling, Effect of surface hydroxyls on CO2 hydrogenation over Cu/γ-Al2O3 catalyst: a theoretical study, J. Phys. Chem. C, 2011, 115, 19811–19818 CAS.
- Y. X. Pan, C. J. Liu and Q. F. Ge, Adsorption and protonation of CO2 on partially hydroxylated γ-Al2O3 surfaces: a density functional theory study, Langmuir, 2008, 24, 12410–12419 CrossRef CAS PubMed.
- M. C. Valero, P. Raybaud and P. Sautet, Nucleation of Pdn (n = 1–5) clusters and wetting of Pd particles on γ-Al2O3 surface: a density functional theory study, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 045427 CrossRef.
- S. Scirè, C. Crisafulli, R. Maggiore, S. Minicò and S. Galvagno, Influence of the support on CO2 methanation over Ru catalysts: an FT-IR study, Catal. Lett., 1998, 51, 41–45 CrossRef.
- R. Wischert, P. Laurent, C. Copéret, F. Delbecq and P. Sautet, γ-Alumina: the essential and unexpected role of water for the structure, stability, and reactivity of “defect” sites, J. Am. Chem. Soc., 2012, 134, 14430–14449 CrossRef CAS PubMed.
- G. Jenness, M. A. Christiansen, S. Caratzoulas, D. Vlachos and R. J. Gorte, Site-dependent Lewis acidity of γ-Al2O3 and its impact on ethanol dehydration and etherification, J. Phys. Chem. C, 2014, 118, 12899–12907 CAS.
- H. Metiu, S. Chrétien, Z. P. Hu, B. Li and X. Y. Sun, Chemistry of Lewis acid–base pairs on oxide surface, J. Phys. Chem. C, 2012, 116, 10439–10450 CAS.
- S. Chrétien and H. Metiu, Enhanced adsorption energy of and on the stoichiometric surface by coadsorption with other molecules, J. Chem. Phys., 2008, 128, 044714 CrossRef PubMed.
- J. Lin, A. Q. Wang, B. T. Qiao, X. Y. Liu, X. F. Yang, X. D. Wang, J. X. Liang, J. Li, J. Y. Liu and T. Zhang, Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction, J. Am. Chem. Soc., 2013, 135, 15314–15317 CrossRef CAS PubMed.
- B. T. Qiao, J. X. Liang, A. Q. Wang, C. Q. Xu, J. Li, T. Zhang and J. Y. Liu, Ultrastable single-atom gold catalysts with strong covalent metal–support interaction (CMSI), Nano Res., 2015, 8, 2913–2924 CrossRef CAS.
- H. S. Wei, X. Y. Liu, A. Q. Wang, L. L. Zhang, B. T. Qiao, X. F. Yang, Y. Q. Huang, S. Miao, J. Y. Liu and T. Zhang, FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes, Nat. Commun., 2014, 5, 5634–5641 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The convergence test for the cutoff energy, the convergence test of the vacuum slab for the (100) and (110) surfaces, the potential energy profiles for the dissociation of H2O on the dehydrated (100) and (110) surfaces, the charge density difference for different configurations of Run (n = 1–4) clusters adsorbed on the (100) and (110) surfaces, the PDOS of atoms of different surfaces involved in Run (n = 1–4) cluster adsorption. See DOI: 10.1039/c5ra28173c |
| This journal is © The Royal Society of Chemistry 2016 |