Diamond@carbon-onion hybrid nanostructure as a highly promising electrocatalyst for the oxygen reduction reaction†
Jaekang Koha,
Sung Hyeon Parkb,
Min Wook Chunga,
Seung Yong Leea and
Seong Ihl Woo*ab
aGraduate School of EEWS, Korea Advanced Institute of Science and Technology, Daejeon 34141, Republic of Korea. E-mail: siwoo@kaist.ac.kr
bDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology, Daejeon 34141, Republic of Korea
First published on 3rd March 2016
Abstract
The modification of nanodiamond derived carbon nano-onions by the formation of additional edge- and defect-sites through the rupturing of surface graphene layers is investigated for its application towards the oxygen reduction reaction (ORR) in acidic media. The catalyst, which had a high degree of edge- and defect-sites on its surface, demonstrated a remarkably enhanced ORR performance compared to that of an edge- and defect-sites poor catalyst, where the onset potential increased from 0.53 to 0.91 V, with a mass activity of 2.70 mA mg−1 (at 0.8 V). According to our electrochemical impedance spectroscopy study, the enhancement in the catalytic performance between the two catalysts could have originated from the charge transfer resistance. Moreover, an accelerated degradation test revealed the outstanding stability of the edge- and defect-rich catalyst, compared to that of Pt/C performed in harsh conditions, which could have originated from the diamond core. The selection of carbon material with adequate modifications to enhance the catalytic activity and stability towards the ORR drafted a scheme for potential catalysts.
1. Introduction
With high energy conversion efficiency and low pollutant production, Polymer Electrolyte Membrane Fuel Cells (PEMFCs) have been highlighted as one of the promising energy conversion devices that could settle the increasing demand for eco-friendly energy devices.1–4 However, due to the high cost of Pt-based catalysts at the electrodes, dilemmas are faced in terms of future mass-scale commercialization. Recently, new catalyst materials, such as N-doped carbons5–7 and M–N–C metal complexes,8,9 have been recognized as promising materials in the area of electrochemistry due to their positive aspects.10 And since then, various carbon materials, such as CNTs, graphene, and mesoporous carbon, have been derived for the substitution of Pt-based catalysts.11–13 The application of tailored nanodiamonds (carbon nano-onions) towards the ORR is at the early stages, but they possess unique properties for applications in electrochemistry.14,15Due to the morphology of the carbon-onion structure, the outermost layer of the nano-onion confines several inner carbon layers from reactants. The limitation of this spherical morphology could be enhanced through the rupturing of the outermost carbon onion layers, exposing the concealed active sites. The construction of edge- and defect-sites at the surface of catalysts has been known to enhance the catalytic activities of N-doped carbons for the ORR.16,17 From our theoretical studies, the doped-N near the edge- and defect-sites enhanced the oxygen adsorption.18 Ozkan et al. mentioned that since the ORR is related to the location of the incorporated N, the N-doped edge- and defect-sites would be the most desirable.19 Moreover, studies have revealed that the electron lone pairs on oxygen molecules favorably bond to the carbons adjacent to the N-doped sites formed at the edge- and defect-sites, where the electronegativity is disrupted.20 Hence, designing catalysts with a high degree of N-doped edge- and defect-sites is presumably desirable for ORR catalysts.
Su et al. has reported that a hybrid structure of graphite at the shell and diamond at the core achieved a synergic effect on stability.15 The graphitized diamond at the shell increases the conductivity for electrochemical reactions, while the remaining sp3-hybridized diamond at the core has been reported to retain thermal, morphological and electrochemical stability.13,14 The harsh operating conditions (>1.1 V) in PEMFCs usually generate issues for their practical application.30 Therefore, improvement in the stability of carbon material through tailoring nanodiamond by preserving a hybrid structure of graphite at the shell and diamond core could be a crucial methodology in designing stable catalysts.
In this study, carbon nano-onions derived from nanodiamonds were modified to enhance both the catalytic activity and stability by generating a synergic effect from the hybrid structure between the two different carbon structures. The diamond was deliberately preserved at the core to retain stability, while modifying the surface carbon layers to enhance catalytic activity within the catalyst material.13,14 To the best of our knowledge, the application of nanodiamond derived carbon based nano-onions with a high ORR performance in acidic media, has yet not been reported in the field of PEMFCs. The prepared catalysts were characterized using transmission electron microscopy (TEM), Raman spectroscopy, EIS and X-ray diffraction (XRD) to analyze the formation of additional edge- and defect-sites, the degree of defect, the charge transfer resistance and the remaining diamond within the catalysts, respectively.
2. Experimental section
2.1 Catalyst synthesis
Nanodiamond powder (10 g, US Research Nanomaterial, Inc., ND) was first purified in 2 M of HCl (36%, Samchun) for 12 h to remove possible impurities. Then the powder was rinsed with de-ionized (DI) water and dried in the oven. Next, the powder was heat-treated at 1550 °C for 6 h under an Ar flow to obtain a carbon nano-onion (hND) sample. The ruptured outer carbon layers (exhND) were gained through exfoliation using the modified Hummer's method from the previous study.2 Precisely 3 g of hND was dispersed in 360 mL of H2SO4 (95%, Samchun) and 40 mL of H3PO4 (85%, Samchun) mixture. 18 g of KMnO4 (99%, Aldrich) was added to the mixture and maintained at 50 °C for 3 h. After cooling to room temperature, the solution was poured into 400 g of ice, followed by the inclusion of 5 mL of H2O2 (34.5%, Samchun), and stirred for 30 min. The mixture was then centrifuged at 8500 rpm for 30 min. The product was washed with 100 mL of HCl (36%, Samchun) and 100 mL of DI-water two times. Afterwards, the exfoliated powder was collected and lyophilized for 1 day. The N-exhND sample was prepared via the pyrolysis of a homogenous mixture of exhND, C2H4N4 (99%, Aldrich, DCDA) and FeSO4·7H2O (99%, Aldrich). Briefly, 0.3 g of exhND powder and 100 mL of DI-water were mixed in a 250 mL flask and dispersed by sonication for 30 min. Then 0.6 g of DCDA and 0.044 g of FeSO4·7H2O were dissolved in the solution, and stirred for 30 min. The solvent was removed using a rotary evaporator (80 °C and 300 mbar) and lyophilized overnight. The obtained powder was heat-treated at 850 °C under an Ar atmosphere. The acid leaching steps of the prepared catalysts were conducted in 0.5 M H2SO4 at 80 °C for 8 h. The powders were collected by centrifugation at 8500 rpm for 30 min, washed with 100 mL of DI-water two times, and lyophilized for 1 day, followed by pyrolysis at 900 °C under an Ar atmosphere. The N-hND sample was prepared with the same method except for the exfoliation step. The N-hND-4 and N-hND-10 catalysts were prepared by an identical procedure to N-hND except for the amount of DCDA added, 2.4 and 6 g, respectively. The N-only catalyst was prepared using the same procedure as the N-exhND without a metal precursor.2.2 Physical characterizations
The catalysts were characterized via XRD, Raman spectroscopy, TEM, XPS, element analysis (EA), and inductively coupled plasma (ICP) spectroscopy. The XRD patterns were acquired using a RIGAKU D/MAX-2500 with 40 kV and 300 mA with a monochromator X-ray source. The patterns were collected from 10–80° (2-theta ranges) with a 0.01° step size and a 1° min−1 scan rate. The Raman spectroscopy data were collected using a LabRAM HR UV/vis/NIR with a 514 nm Ar ion CW laser source. The TEM images were taken using a JEM2100-F (Jeol Ltd.) operated at 200 kV. The XPS peaks were collected from a Sigma Probe (Thermo VG Scientific) equipped with a microfocused monochromator X-ray source. EA analysis for the measurement of C, H, N, S and O was performed on a Flash EA 1112, and a POLYSCAN 61 E (Thermo Jarell Ash) was used for the ICP analysis of Fe content.2.3 Electrochemical characterizations
All potential used in this experiment is converted to vs. reversible hydrogen electrode (vs. RHE). All electrochemical experiments were performed using a CHI700D (CH Instrument Inc.) and a rotating ring disk electrode 3A (ALS Co., RRDE) electrode, Ag/AgCl reference electrode (012167 ALS Co.) and Pt wire counter electrode (002233 ALS Co.). The prepared catalyst (10 mg) was dispersed in 1 mL of Nafion ink solution (5 wt% Nafion in water and 2-propanol) and 5 μL of ink solution was dropped onto the glassy carbon (4 mm) of RRDE. The loading amount of the catalyst was 398 μg cm−2. The ink was dried in a vacuum oven for 10 min. In acidic conditions, cyclic voltammetry (CV) was performed for the activation process with the range of 1.22 to 0 V and a scan rate of 40 mV s−1 for 100 cycles in N2-bubbled 0.1 M HClO4 electrolyte to remove unstable species at the surface of the catalyst in case they exist. The linear sweep voltammetry (LSV) with a potential range of 1.04 to 0.14 V with a 5 mV s−1 scan rate was performed in both N2- and O2-bubbled 0.1 M HClO4 electrolytes. The capacitance current from the N2-bubbled 0.1 M HClO4 electrolyte was subtracted from the current measured in the O2-bubbled electrolyte of 0.1 M HClO4. The loading of the Pt/C (20 wt%, Johnson Matthey) catalyst on the RRDE was 20 μgPt cm−2. A Tafel-plot calculation was obtained from the following equation (eqn (1)).
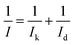 | (1) |
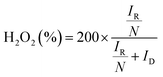 | (2) |
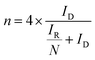 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz at 5 mV amplitude. The system was fabricated with 3 mg of active materials separated by a glass filter paper (0.22 μm pore diameter, Durapore membrane filter) in 0.1 M HClO4 as an electrolyte.
000 Hz at 5 mV amplitude. The system was fabricated with 3 mg of active materials separated by a glass filter paper (0.22 μm pore diameter, Durapore membrane filter) in 0.1 M HClO4 as an electrolyte.
3. Results and discussion
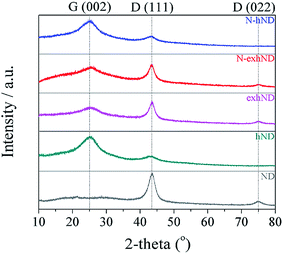
The experimental procedures with the designated nomenclature of the prepared catalysts are shown in Fig. 1. Moreover, the presumed morphologies of the prepared catalysts after each process were observed using TEM (Fig. 2). The commercial ND had a spherical morphology with diameter variations between 3 to 10 nm. When the ND was heat-treated at a high temperature, a transformation into nano-sized onion like spheres with few layers of graphitic shells was observed, which is in agreement with the reported literature.21,22 The diameter of the synthesized carbon nano-onion particles varied between 5 and 10 nm. After the exfoliation step, the rupture of the outermost layers could be seen, creating various edge- and defect-sites at the peel of the onion-like carbons. The samples N-exhND and N-hND had similar surface morphologies to their preceding samples (exhND and hND, respectively), implying that the exfoliation was a crucial step in producing the abundant edge- and defect-sites on the catalyst surface in N-exhND.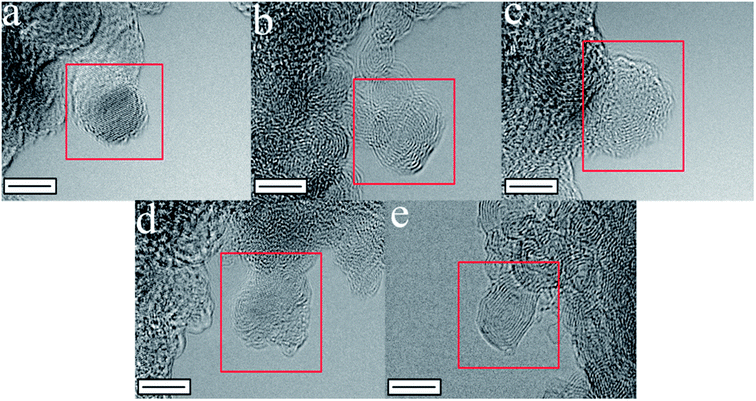 | ||
| Fig. 2 The representative TEM images (5 nm scale) of (a) ND, (b) hND, (c) exhND, (d) N-exhND, and (e) N-hND. | ||
In all of the prepared catalysts, the remaining diamond could be detected at the peaks assigned for diamond, (111) and (022), as confirmed from the XRD analysis, and consistent with the reported literature.23 Except for the ND sample, the prepared catalysts had peaks at 25°, assigned for the (002) graphitic peak, inferring the formation of graphitic carbon after heat treatment (Fig. 3), similar to other studies.24 The intensity of graphitic (002) peaks in exhND and N-exhND catalysts reduced after the exfoliation step from hND, which supports indirectly the formation of defect sites. The increase in the degree of defect was also observed from the ID/IG ratio calculated from the Raman spectroscopy. The prepared catalysts showed G- and D-bands from the E2g vibrational mode in the highly oriented pyrolytic graphite (HOPG) and the lattice distortion in sp2-hybridized carbon, respectively (Fig. 4).25 Thus, the degree of defect can be implied by calculating the ratio of the two intensities (ID/IG). The ID/IG ratio of the prepared catalysts increased after exfoliation from 0.89 (hND) to 1.06 (exhND) which is assumed to be related to the development of defects. Small increases of the ID/IG ratio were also found after further modifications (N-doping) due to the impregnation of heteroatoms in the carbon lattices that could induce additional defect sites.26 And as assumed, the N-hND catalyst demonstrated a lower ID/IG ratio compared to N-exhND, advocating that the defect formation was caused mostly by exfoliation rather than from N-doping. Moreover, the 2D-bands at 2670 cm−1, suggesting stacked graphitic layers, appeared only in the hND and N-hND catalysts, indirectly resulting in a lower degree of defect compared to those of the exhND and N-exhND catalysts.
Lim et al. proposed that a superior electron transfer rate was observed on the edge- and defect-sites rather than on the central basal plane of the graphene.27 Moreover, Yin et al. implied that the decrease in the charge transfer resistance of the catalyst increased the ORR catalytic activity.28 Hence, EIS was analyzed to measure the charge transfer kinetics of the prepared catalysts. A home-made symmetric cell was used in order to rule out the side electrochemical reactions.29 Fig. 5a and S2† show the Nyquist plot and the obtained parameter values by the simulation of the prepared catalyst operated between the ranges of 0.1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz. The vertical shapes at the lower frequencies imply low ionic diffusion resistance, while the high frequency region indicates the low electronic resistance of the prepared catalyst.30,31 The charge transfer resistance (RCT) of N-exhND had a smaller semi-circle than that of N-hND. Both N-exhND and N-hND catalysts appeared to have vertical shapes at the lower frequency region which express no compelling contrast in ionic diffusion resistance, implying that the N-hND catalyst shows a higher difference in charge transfer resistance rather than in ionic diffusion resistance compared to the N-exhND catalyst.
000 Hz. The vertical shapes at the lower frequencies imply low ionic diffusion resistance, while the high frequency region indicates the low electronic resistance of the prepared catalyst.30,31 The charge transfer resistance (RCT) of N-exhND had a smaller semi-circle than that of N-hND. Both N-exhND and N-hND catalysts appeared to have vertical shapes at the lower frequency region which express no compelling contrast in ionic diffusion resistance, implying that the N-hND catalyst shows a higher difference in charge transfer resistance rather than in ionic diffusion resistance compared to the N-exhND catalyst.
The composition and deconvolution of N in the prepared catalysts were organized using EA and XPS in Table S1 and Fig. S3.† The bulk (1.92 at%) and surface compositions (7.32 at%) of N in N-exhND were higher than those in N-hND (both <1 at%), although the deconvolution of the XPS-N1s of N-exhND assigned for pyridinic N (398.2 eV), graphitic N (400.9 eV) and pyridinic oxide (403.6 eV) was 54.9, 35.0 and 10.1%, respectively. The XPS-N1s for N-hND and the XPS-Fe2p for both N-exhND and N-hND were unattainable due to the detection limit (<1 at%). The same amount of Fe and N precursors was used in the catalyst preparation, however the amount of N-doping was different. Thus, the doping of N could be seen to be favorable at the edge- and defect-sites rather than on the basal plane.
In Fig. 5b, the catalysts ND, hND and exhND showed low catalytic activities towards the ORR. However, after N-doping on hND and exhND, a significant enhancement in the ORR performance was observed. The measured onset potentials for N-exhND and N-hND from the Tafel plot in Fig. 5c and S4† were 0.91 and 0.53 V, respectively. The difference in the onset potentials between Pt/C (0.97 V) and the N-exhND catalysts was 0.06 V, with a half-wave potential difference of 51 mV. The mass activities calculated from the Tafel plot at 0.8 V for N-exhND and Pt/C catalyst were 2.70 and 6.82 mA mg−1, respectively. The mass activity of the N-hND sample could not be measured at 0.8 V due to its low catalytic activity (Fig. S4†). The measured current densities of N-exhND and Pt/C at 0.8 V were −0.84 and −1.57 mA cm−2, respectively. Even though the current density of N-exhND was about 54% of that of Pt/C, the N-exhND catalyst demonstrated a remarkably higher ORR performance compared to that of N-hND at the chosen potential.
As the amount of N-doping differs between N-hND and N-exhND, a catalyst with higher N-doping (named N-hND-10) was prepared for comparison and is shown in Fig. S6 and Table S2,† but these catalysts showed far less ORR activity compared to N-exhND. To identify the nature of ORR catalytic activity, N-exhND catalysts were synthesized without metal, and so called N-only. Interestingly, a much higher ORR performance was shown compared to N-hND-10, which revealed that the nitrogen doped on edge- and defect-sites has a high possibility of being an active site towards the ORR, in the case of our catalyst (Fig. S7a†). However, it still demonstrated less catalytic performance towards N-exhND, where it is also doubtful that the metal is an active site. In order to verify the contribution of metal involved in the ORR performance, the CN− poisoning test was conducted as shown in Fig. S7b.† From the CN− poisoning test, no significant performance drop was found. However, a minor decrease was shown in the ORR currents, which also can be seen with the KNO3 containing electrolyte (Fig. S7c†). The same decrease of the performance in the KNO3 containing electrolyte proves that the minor decrease was caused by the effect of ionic species near the electrode.2,29 Thus, the ORR catalytic activity mainly originates from N-doped carbon sites. Moreover, it is quite difficult to show that the metal exists on the surface of the catalyst, as shown in the Fe XPS peak (Fig. S3b†). This could suggest that the metal could be embedded inside of the catalyst structure and changes the work function of the catalyst resulting in a higher performance towards the ORR.2
Concerning the pathway of the ORR of N-exhND and N-hND, the H2O2 production yield was calculated as shown in Fig. 6a. The difference in the distribution of the edge- and defect-sites on the catalyst showed no significant influence on H2O2 production. The electron numbers per oxygen molecule in the ORR were calculated for N-exhND (4.00) and N-hND (3.82). However, the RRDE catalyst loading might have an impact on the H2O2 production yield during the ORR as reported in the literature,32,33 and the various amounts of loadings were also examined with the N-exhND catalyst as shown in Fig. S9.† In the case of our catalyst, the H2O2 productions remain very low in all loadings, which shows that the ORR on the catalyst proceeds mostly according to a direct four electron mechanism and a similar result was found in reported literature as well.34 The synthesized catalysts were driven near the 4-electron pathway, producing low amounts of H2O2, which is desirable for fuel cell stability.
The stability tests for the N-exhND and Pt/C catalysts were conducted via ADT, during 5000 cycles with a 500 mV s−1 scan rate. The potential range was set between 0.6 and 1.4 V, since the carbon oxidation below 1.0 V is slow but increases drastically above 1.2 V.35 The changes in the ORR performances of the prepared catalysts, before and after the ADT analysis, are shown in Fig. 6c and d. The differences in the half-wave potential for N-exhND and Pt/C catalysts were 31 and 133 mV, respectively. The current densities measured at 0.8 V for N-exhND decreased from −0.84 to −0.61 mA cm−2, with a 27% performance decrease. In the case of the commercial Pt/C, the current density decreased from −1.57 to −0.32 mA cm−2, which is an 80% performance decrease. The higher stability of the N-exhND catalyst compared to that of Pt/C might be due to the presence of the sp3-hybridized carbon remaining at the core.14,15 Furthermore, the result for the ADT analysis was conducted in extremely severe conditions even compared to the other reported studies (Table S3†). The methanol tolerance was examined by injecting 1 mL of methanol at the selected time during the chronoamperometric analysis in 0.1 M HClO4 at the applied constant potential of 0.6 V. As shown in Fig. 6b, the N-exhND catalyst exhibited a strong methanol tolerance compared to that of the Pt/C catalyst.
Recent studies have suggested the importance of designing edge- and defect-sites on the surface of the carbon-based catalysts for high catalytic activity towards the ORR.36–38 In our experiment, the additional exfoliation process created a higher degree of defect sites at the surface, which was confirmed by TEM and Raman spectroscopy. The ORR activity of the prepared catalyst demonstrated a notable performance difference between N-exhND and N-hND catalysts, which advocates that the higher density of edge- and defect-sites produced from the exfoliation step has a positive effect on the ORR performance. As stated by Brownson et al., the electrons with the highest energies, and therefore most likely to transfer, have a higher probability of being concentrated in the edge- and defect-sites than in the basal plane.39 Hence, N-exhND having a higher degree of edge- and defect-sites, and therefore having high energy electrons concentrated near the region, could have led to a higher performance in the ORR compared to that of N-hND. In addition, our EIS results show a lower charge resistance in the N-exhND compared to that of the N-hND catalyst. Thus, the high catalytic activity could be acquired from the higher charge density formed in N-exhND than in N-hND.
4. Conclusions
In summary, a series of catalysts was synthesized through the physical and chemical modifications of nanodiamonds. The hybrid structure of the graphitized diamond, N-doped edges at the shell and sp3-hybridized diamond structure at the core, conceived a synergic effect with a considerably enhanced ORR activity and outstanding stability within the N-exhND catalyst. The N-exhND exhibited an onset potential of 0.91 V while that of N-hND was 0.53 V. This increase could be due to the modification of the catalyst surface, which decreased the charge transfer resistance, as shown by the EIS. The mass activity calculated from the Tafel plot for the N-exhND catalyst was 2.70 mA mg−1 at 0.8 V, which is about 40% of that of Pt/C (6.82 mA mg−1). Moreover, the N-exhND retained a performance of 73% after 5000 cycles of ADT, which demonstrated a higher stability than that of Pt/C (20%). The synthetic strategy, the modification of the catalyst surface with a higher degree of edge sites and the preservation of the diamond structure at the core, demonstrated new insights that could become a guideline for the design of highly promising catalysts for the ORR.Acknowledgements
This work was supported by grant No. EEWS-2014-N01140048 from the Climate Change Research Hub Project of the KAIST EEWS Research Center. (EEWS: Energy, Environment, Water and Sustainability).Notes and references
- G. Wu, K. L. More, C. M. Johnston and P. Zelenay, Science, 2011, 332, 443–447 CrossRef CAS PubMed.
- C. H. Choi, H. K. Lim, M. W. Chung, J. C. Park, H. Shin, H. Kim and S. I. Woo, J. Am. Chem. Soc., 2014, 136, 9070–9077 CrossRef CAS PubMed.
- Y. Wang, K. S. Chen, J. Mishler, S. C. Cho and X. C. Adroher, Appl. Energy, 2011, 88, 981–1007 CrossRef CAS.
- J. Sanetuntikul, T. Hang and S. Shanmugam, Chem. Commun., 2014, 50, 9473–9476 RSC.
- B. Jeong, D. Shin, H. Jeon, J. D. Ocon, B. S. Mun, J. Baik, H.-J. Shin and J. Lee, ChemSusChem, 2014, 7, 1289–1294 CrossRef CAS PubMed.
- G. Liu, X. G. Li, P. Ganesan and B. N. Popov, Electrochim. Acta, 2010, 55, 2853–2858 CrossRef CAS.
- L. F. Lai, J. R. Potts, D. Zhan, L. Wang, C. K. Poh, C. H. Tang, H. Gong, Z. X. Shen, L. Y. Jianyi and R. S. Ruoff, Energy Environ. Sci., 2012, 5, 7936–7942 CAS.
- E. Negro, A. H. A. M. Videla, V. Baglio, A. S. Arico, S. Specchia and G. J. M. Koper, Appl. Catal., B, 2015, 166, 75–83 CrossRef.
- Y. S. Zhu, B. S. Zhang, X. Liu, D. W. Wang and D. S. Su, Angew. Chem., Int. Ed., 2014, 53, 10673–10677 CrossRef CAS PubMed.
- Q. Wei, X. Tong, G. Zhang, J. Qiao, Q. Gong and S. Sun, Catalysts, 2015, 5, 1574 CrossRef CAS.
- X.-J. Huang, Y.-N. Luan, P.-F. Yao, J.-S. Xie, L. Yu, Z.-Y. Wu and P. Chen, RSC Adv., 2015, 5, 76599–76606 RSC.
- J. Wei, Y. Hu, Y. Liang, B. Kong, J. Zhang, J. Song, Q. Bao, G. P. Simon, S. P. Jiang and H. Wang, Adv. Funct. Mater., 2015, 25, 5768–5777 CrossRef CAS.
- J. Wei, Y. Hu, Z. Wu, Y. Liang, S. Leong, B. Kong, X. Zhang, D. Zhao, G. P. Simon and H. Wang, J. Mater. Chem. A, 2015, 3, 16867–16873 CAS.
- Y. J. Wang, J. B. Zang, L. Dong, H. Pan, Y. G. Yuan and Y. H. Wang, Electrochim. Acta, 2013, 113, 583–590 CrossRef CAS.
- L. Dong, J. B. Zang, Y. J. Wang, H. Pan, Y. H. Wang, Y. L. Zhao and J. Su, J. Electrochem. Soc., 2014, 161, F185–F191 CrossRef CAS.
- T. Sharifi, G. Hu, X. E. Jia and T. Wagberg, ACS Nano, 2012, 6, 8904–8912 CrossRef CAS PubMed.
- G. Y. Zhong, H. J. Wang, H. Yu and F. Peng, Electrochem. Commun., 2014, 40, 5–8 CrossRef CAS.
- H. Kim, K. Lee, S. I. Woo and Y. Jung, Phys. Chem. Chem. Phys., 2011, 13, 17505–17510 RSC.
- E. J. Biddinger and U. S. Ozkan, J. Phys. Chem. C, 2010, 114, 15306–15314 CAS.
- W. H. He, C. H. Jiang, J. B. Wang and L. H. Lu, Angew. Chem., Int. Ed., 2014, 53, 9503–9507 CrossRef CAS PubMed.
- A. Krueger and D. Lang, Adv. Funct. Mater., 2012, 22, 890–906 CrossRef CAS.
- J. Xiao, G. Ouyang, P. Liu, C. X. Wang and G. W. Yang, Nano Lett., 2014, 14, 3645–3652 CrossRef CAS PubMed.
- J. Qian, C. Pantea, J. Huang, T. W. Zerda and Y. Zhao, Carbon, 2004, 42, 2691–2697 CrossRef CAS.
- Q. Liu, H. Zhang, H. Zhong, S. Zhang and S. Chen, Electrochim. Acta, 2012, 81, 313–320 CrossRef CAS.
- C. H. Choi, S. H. Park and S. I. Woo, Appl. Catal., B, 2012, 119, 123–131 CrossRef.
- C. H. Choi, M. W. Chung, Y. J. Jun and S. I. Woo, RSC Adv., 2013, 3, 12417–12422 RSC.
- C. X. Lim, H. Y. Hoh, P. K. Ang and K. P. Loh, Anal. Chem., 2010, 82, 7387–7393 CrossRef CAS PubMed.
- H. Yin, C. Zhang, F. Liu and Y. Hou, Adv. Funct. Mater., 2014, 24, 2930–2937 CrossRef CAS.
- M. W. Chung, C. H. Choi, S. Y. Lee and S. I. Woo, Nano Energy, 2015, 11, 526–532 CrossRef CAS.
- G. Cai, X. Wang, M. Cui, P. Darmawan, J. Wang, A. L.-S. Eh and P. S. Lee, Nano Energy, 2015, 12, 258–267 CrossRef CAS.
- G. Huang, C. Hou, Y. Shao, B. Zhu, B. Jia, H. Wang, Q. Zhang and Y. Li, Nano Energy, 2015, 12, 26–32 CrossRef CAS.
- E. J. Biddinger, D. von Deak, D. Singh, H. Marsh, B. Tan, D. S. Knapke and U. S. Ozkan, J. Electrochem. Soc., 2011, 158, B402–B409 CrossRef CAS.
- A. Bonakdarpour, M. Lefevre, R. Z. Yang, F. Jaouen, T. Dahn, J. P. Dodelet and J. R. Dahn, Electrochem. Solid-State Lett., 2008, 11, B105–B108 CrossRef CAS.
- V. Goellner, V. Armel, A. Zitolo, E. Fonda and F. Jaouen, J. Electrochem. Soc., 2015, 162, H403–H414 CrossRef CAS.
- K. Nagasawa, S. Takao, K. Higashi, S. Nagamatsu, G. Samjeske, Y. Imaizumi, O. Sekizawa, T. Yamamoto, T. Uruga and Y. Iwasawa, Phys. Chem. Chem. Phys., 2014, 16, 10075–10087 RSC.
- A. Serov, K. Artyushkova and P. Atanassov, Adv. Energy Mater., 2014, 4, 1301735 Search PubMed.
- A. Shen, Y. Zou, Q. Wang, R. A. W. Dryfe, X. Huang, S. Dou, L. Dai and S. Wang, Angew. Chem., Int. Ed., 2014, 53, 10804–10808 CrossRef CAS PubMed.
- Y. Zhao, C. G. Hu, L. Song, L. X. Wang, G. Q. Shi, L. M. Dai and L. T. Qu, Energy Environ. Sci., 2014, 7, 1913–1918 CAS.
- D. A. C. Brownson, L. J. Munro, D. K. Kampouris and C. E. Banks, RSC Adv., 2011, 1, 978–988 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra28066d |
| This journal is © The Royal Society of Chemistry 2016 |