Quantum chemical approach for highly durable anion exchange groups in solid-state alkaline fuel cells†
K. Matsuyamaa,
H. Ohashia,
S. Miyanishia,
H. Ushiyamab and
T. Yamaguchi*a
aChemical Resources Laboratory, Tokyo Institute of Technology, R1-17 4259 Nagatsuta-cho, Midori-ku, Yokohama, Kanagawa 226-8503, Japan. E-mail: yamag@res.titech.ac.jp; Fax: +81-45-924-5253; Tel: +81-45-924-5254
bDepartment of Chemical System Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
First published on 7th April 2016
Abstract
Durable anion exchange groups are required for solid-state alkaline fuel cells. In the present study, the lowest unoccupied molecular orbital (LUMO) was employed as a design guideline for durable anion exchange groups. The correlation between the LUMO and the durability of the anion exchange group was clarified.
1. Introduction
Fuel cells are eco-friendly and highly efficient devices. Proton exchange membrane fuel cells (PEMFCs) are promising candidates of energy production for portable energy devices at low temperatures; however the acid environment in PEMFCs mostly limits the catalysts and fuels to precious metals and hydrogen gas. On the other hand, alkaline fuel cells (AFCs) can make use of non-precious metals as catalysts1–4 because unlike acid environments, alkali conditions do not erode most metals. Moreover, liquid fuels with high energy densities, such as methanol,5,6 ammonia,7,8 and hydrazine,9,10 can be used in AFCs due to the broadened range of usable catalysts. However, since free alkali (e.g., potassium hydroxide) is used as an electrolyte, the precipitation of free alkali itself or carbonate due to the reaction with carbon dioxide leads to a decrease in performances. Recently, solid-state alkaline fuel cells (SAFCs) introducing anion exchange membrane as solid-state alkaline into PEFCs have been focused on.11–13 SAFCs can acquire the merits of AFCs and do not require free alkali causing the problematic carbonate because anion exchange groups of solid-state alkali conduct hydroxide ions in the devices.For the practical use of SAFCs, it is necessary to develop anion exchange membranes to conduct hydroxide ion. However, the development of these membranes remains in the early stages, and hydroxide ions, conducting species, decompose the membrane due to the nucleophilicity of the hydroxide ion.14–16 The decomposition of anion exchange groups results in decreases in membrane conductivity and SAFC performance. To overcome this problem, various anion exchange groups have been synthesized, and their stabilities have been discussed.15 However, experimental investigations of the stabilities of all anion exchange group candidates are time-consuming and inefficient. Thus, refining the possible groups using systematic calculations is desirable.
As an example of refinement based on calculation, the oxidative decomposition rates of various main chains of electrolyte polymers for proton exchange membrane fuel cells were correlated with their highest occupied molecular orbital (HOMO) energies, indicating that the molecular orbital information can serve as a design guideline.17 The activation energy for the decomposition reaction with hydroxide ions was also calculated and considered in relation to the chemical stabilities of anion exchange groups.18–20 These past studies adequately described the detailed degradation pathways; however, they could not efficiently screen durable anion exchange groups due to the difficulty and computational cost associated with searching for the transition states. Thus, a facile and general method to determine the stabilities of anion exchange groups is needed.
The hydroxide ion is strong nucleophile, making it reactive toward various molecules. Because the reactivity is correlated with vacant orbitals in the molecular orbitals, lowest unoccupied molecular orbital (LUMO) was focused on in the present study. The LUMO primarily undergoes nucleophilic attack since it has the lowest energy of all unoccupied molecular orbitals. A previous study found a relationship between the stabilities of anion exchange groups and their LUMOs, although the targets were limited to one series of anion exchange groups (e.g., imidazolium or guanidinium).19,21 No study has comprehensively considered the LUMOs of various anion exchange groups. In the present study, we extended the concept and assumed that LUMO can serve as a general guideline for designing durable anion exchange groups. Also, the previous researches focused on only LUMO energy, and there are no research which focuses on the molecular orbitals shape of hydroxide ion and anion exchange groups. Therefore, the relationship between the experimental durabilities of model molecules that have various anion exchange groups and their LUMO energies and shapes was investigated. The correlation between the LUMO isosurface and anion exchange group stability was also studied.
2. Methods
2.1. Selection of target molecules
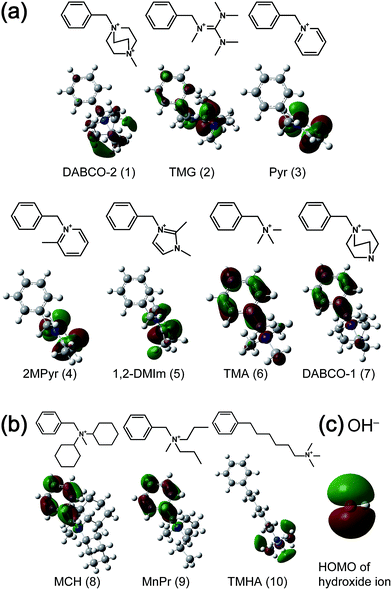
In a previous study,22 various anion exchange groups were substituted at the benzylic position in benzene. After the counter anions of these molecules were converted to a hydroxide ion by deuterated water (D2O) and silver oxide (Ag2O), their stabilities were investigated at 60 °C and/or 120 °C in D2O. Seven of these molecules that were investigated at 60 °C were chosen for investigation in the present study and DABCO-1 (7) [Fig. 1(a)] was not decomposed at 60 °C. Three additional molecules that decomposed more slowly than DABCO-1 (7) at 120 °C were chosen as durable model molecules [Fig. 1(b)]: (8) N-methyl-N,N-dicyclohexylbenzylammonium (MCH); (9) N-methyl-N,N-dipropylbenzylammonium (MnPr) and (10) N,N,N-trimethyl-6-phenylhexylaminium (TMHA). Thus, ten molecules were chosen in total (Fig. 1).2.2. Extractions of degradation rate
The degradation rates of model molecules in the presence of hydroxide ions were extracted from the ratios of the remaining (1)–(7) after degradation tests at 60 °C. Because the degradation rates changed over time,22 the ratios of the molecules remaining after 144 h, representing a relatively early stage of the reaction, were adopted for the extractions of the degradation rate. Moreover, because model molecules (8)–(10) decomposed more slowly than DABCO-1 (7) at 120 °C, their degradation rates at 60 °C were regarded as 0% h−1.2.3. Calculation of molecular orbitals
The calculations conducted in the present study were second-order perturbation (MP2) calculations performed using the Gaussian09 package program23 with the 6-311G(d,p) basis set with geometry optimization. To investigate the electronic structures, the LUMOs of the model molecules and the HOMO of the hydroxide ion were calculated. Their energies and isosurfaces were also determined.3. Results and discussion
3.1. Investigation of model molecule durability
The hydroxide ion is an electron-rich nucleophile and therefore interacts with the vacant orbitals of model molecules. Because the LUMO has the lowest energy of all of the vacant orbitals, the LUMO can more easily accept an electron compared with other vacant orbitals. Moreover, the electron in the HOMO has the highest energy of all the occupied orbitals. Thus, an electron can be transferred from the HOMOs of the hydroxide ions to the LUMOs of the model molecules. Fig. 1 shows the HOMO isosurface of hydroxide ion and the LUMO isosurfaces of the model molecules. Fig. 2 shows the relationship between the LUMO energy and the degradation rate of the model molecules.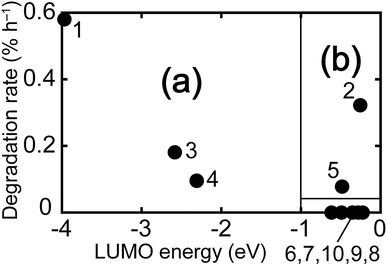 | ||
| Fig. 2 The relationship between LUMO energy and degradation rate.22 | ||
3.2. The relationship between LUMO energy and degradation rate
The model molecules with low LUMO energies decomposed relatively quickly [i.e., (1), (3), and (4) in Fig. 2(a)]. For instance, although the structures of DABCO-1 (9) and DABCO-2 (1) are similar, their LUMO energies are greatly different (−0.49 eV for DABCO-1 vs. −4.0 eV for DABCO-2), causing DABCO-2 (1) to decompose faster.This indicates that a molecule's degradation rate largely depends on its electronic state. When a hydroxide ion and the model molecules interact (Fig. 3), a lower LUMO energy of the model molecule corresponds to a more stabilized electron energy. As a result, the model molecules with low LUMO energies decomposed easily. A previous study limited to only one series of anion exchange groups indicated a relationship between the LUMO energy and degradation rate of the molecules.21,24 The results of this study suggest that this trend can be applied to a variety of anion exchange groups.
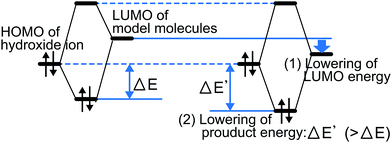 | ||
| Fig. 3 Schematic showing the relationship between the LUMO energy and reactivity of model molecules. | ||
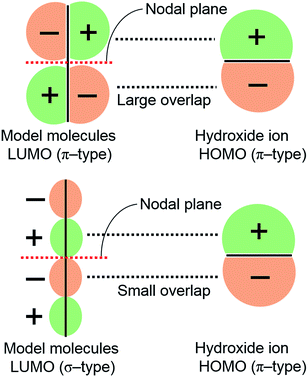
3.3. The relationship between LUMO isosurface and degradation rate
Some molecules decomposed despite their high LUMO energies [model molecules (2) and (5) in Fig. 2(b)]. To explain this phenomenon, the relationship between the LUMO isosurface and degradation rate of these molecules was considered. Model molecules (2)–(5) have π-type LUMOs localized on the anion exchange groups. The HOMO isosurface of hydroxide ion is also π-type [Fig. 1(c)]. The fast degradation can be attributed to the overlap between the LUMO of the anion exchange group and the HOMO of hydroxide ion. As shown in Fig. 4, the overlap and the interaction between π–π orbitals is larger than those between π–σ orbitals. This explains why model molecules with high LUMO energies with π-type LUMO on anion exchange groups, such as TMG (2) and 2MPyr (5), decomposed quickly.As one of the evidence of the above discussion, model molecules with π-type LUMO on anion exchange groups such as guanidinium or imidazolium can be also found in the previous study,19,25 and the anion exchange groups of the molecules decomposed fast. This fact also suggested that LUMO shape has correlation with the degradation under alkaline condition.
The LUMO of TMHA (10) is localized on an anion exchange group, but its LUMO is high in energy and is not π-type. Thus, the interaction of TMHA (10) with the hydroxide ion is weak, resulting in a slow degradation rate.
In the present study, model molecules with more durable anion exchange groups, which can be decomposed only at higher temperature (120 °C), were also investigated in ESI.† Clear tendency cannot be confirmed for the relationship between degradation rate at 120 °C and LUMO energies/shapes as shown in Fig. S1 and S2,† suggesting that the other dominant factors such as steric and/or solvation effects may be dominant in such harsh conditions.
The above results suggest that the durability of an anion exchange groups depends on the LUMO energy and isosurface. Durable anion exchange groups require (1) high LUMO energy (more than −1 eV) and (2) a LUMO that is not π-type and is not localized on anion exchange groups. All the model molecules investigated in the present study satisfy these two requirements. The guidelines developed herein are applicable to the further development of durable anion exchange membranes for SAFCs.
4. Conclusions
The durabilities of model molecules with various anion exchange groups in the presence of hydroxide ion were investigated based on LUMOs. The model molecules with low LUMO energies (less than −1 eV) were unstable. The model molecules with π-type LUMOs localized on anion exchange groups were also unstable, indicating that LUMO isosurfaces are related to degradation rate. By removing such molecules in the design phase, the burden of the successive synthesis phase will be substantially decreased.It should be noted that bulky protecting groups were reported to improve durabilities of anion exchange groups.21,26 The present research does not include molecules having protecting groups. The new design guideline would be required to consider such steric effects. For example, the durability of protecting groups themselves could be discussed by LUMO.
Acknowledgements
The numerical calculations were conducted on the TSUBAME2.5 supercomputer at the Tokyo Institute of Technology, Tokyo, Japan. This work was conducted under the national project on solid-state alkaline fuel cells, and the financial support from JST-CREST project is greatly acknowledged. The authors would like to thank Enago (http://www.enago.jp) for the English language review.Notes and references
- X. G. Li, B. N. Popov, T. Kawahara and H. Yanagi, J. Power Sources, 2011, 196, 1717–1722 CrossRef CAS.
- J. S. Spendelow and A. Wieckowski, Phys. Chem. Chem. Phys., 2007, 9, 2654–2675 RSC.
- Q. G. He, X. F. Yang, X. M. Ren, B. E. Koel, N. Ramaswamy, S. Mukerjee and R. Kostecki, J. Power Sources, 2011, 196, 7404–7410 CrossRef CAS.
- R. Z. Zhang and W. Chen, J. Mater. Chem. A, 2013, 1, 11457–11464 CAS.
- E. J. Cairns and D. C. Bartosik, J. Electrochem. Soc., 1964, 111, 1205–1210 CrossRef CAS.
- C. W. Xu, L. Q. Cheng, P. K. Shen and Y. L. Liu, Electrochem. Commun., 2007, 9, 997–1001 CrossRef CAS.
- E. J. Cairns, E. L. Simons and A. D. Tevebaug, Nature, 1968, 217, 780–781 CrossRef CAS.
- D. W. Mckee, A. J. Scarpell, I. F. Danzig and M. S. Pak, J. Electrochem. Soc., 1969, 116, 562–568 CrossRef CAS.
- K. Tamura and T. Kahara, J. Electrochem. Soc., 1976, 123, 776–780 CrossRef CAS.
- G. E. Evans and K. V. Kordesch, Science, 1967, 158, 1148–1152 CAS.
- H. M. Jung, K. Fujii, T. Tamaki, H. Ohashi, T. Ito and T. Yamaguchi, J. Membr. Sci., 2011, 373, 107–111 CrossRef CAS.
- J. R. Varcoe, R. C. T. Slade and E. Lam How Yee, Chem. Commun., 2006, 1428–1429, 10.1039/b600838k.
- Y. S. Ye, S. Sharick, E. M. Davis, K. I. Winey and Y. A. Elabd, ACS Macro Lett., 2013, 2, 575–580 CrossRef CAS.
- J. Miyake, M. Watanabe and K. Miyatake, Polym. J., 2014, 46, 656–663 CrossRef CAS.
- M. R. Hibbs, J. Polym. Sci., Polym. Phys. Ed., 2013, 51, 1736–1742 CrossRef CAS.
- A. Amel, L. Zhu, M. Hickner and Y. Ein-Eli, J. Electrochem. Soc., 2014, 161, F615–F621 CrossRef CAS.
- J. Lawrence, K. Yamashita and T. Yamaguchi, J. Power Sources, 2015, 279, 48–54 CrossRef CAS.
- H. Long, K. Kim and B. S. Pivovar, J. Phys. Chem. C, 2012, 116, 9419–9426 CAS.
- W. W. Li, S. B. Wang, X. F. Zhang, W. P. Wang, X. F. Xie and P. C. Pei, Int. J. Hydrogen Energy, 2014, 39, 13710–13717 CrossRef CAS.
- S. Chempath, B. R. Einsla, L. R. Pratt, C. S. Macomber, J. M. Boncella, J. A. Rau and B. S. Pivovar, J. Phys. Chem. C, 2008, 112, 3179–3182 CAS.
- B. C. Lin, H. L. Dong, Y. Y. Li, Z. H. Si, F. L. Gu and F. Yan, Chem. Mater., 2013, 25, 1858–1867 CrossRef CAS.
- A. D. Mohanty and C. Bae, J. Mater. Chem. A, 2014, 2, 17314–17320 CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, N. J. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- W. P. Wang, S. B. Wang, X. F. Xie, Y. F. Lv and V. Ramani, Int. J. Hydrogen Energy, 2014, 39, 14355–14361 CrossRef CAS.
- W. P. Wang, S. B. Wang, X. F. Xie, Y. F. Lv and V. K. Ramani, J. Membr. Sci., 2014, 462, 112–118 CrossRef CAS.
- K. M. Hugar, H. A. Kostalik and G. W. Coates, J. Am. Chem. Soc., 2015, 137, 8730–8737 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The relationship between the degradation rate at 120 °C and LUMO energy and shapes of model molecules. See DOI: 10.1039/c5ra27939a |
| This journal is © The Royal Society of Chemistry 2016 |