Thermally induced chemical cross-linking reinforced fluorinated polyurethane/polyacrylonitrile/polyvinyl butyral nanofibers for waterproof-breathable application†
Junlu Shenga,
Min Zhanga,
Wenjing Luob,
Jianyong Yu*ac and
Bin Ding*ac
aKey Laboratory of Textile Science & Technology, Ministry of Education, College of Textiles, Donghua University, Shanghai 201620, China. E-mail: binding@dhu.edu.cn; yujy@dhu.edu.cn
bDepartment of Occupational and Environmental Health, School of Public Health, Fourth Military Medical University, Xi'an, Shanxi 710032, China
cNanofibers Research Center, Modern Textile Institute, Donghua University, Shanghai 200051, China
First published on 18th March 2016
Abstract
Electrospun nanofibrous membranes with thin fiber diameter, small pore size, and high porosity have attracted a great deal of attention in the waterproof and breathable field. However, great challenges still remain in simultaneously reinforcing the mechanical and waterproof-breathable performance of such materials. In this study, a new type of fluorinated polyurethane/polyacrylonitrile/polyvinyl butyral nanofibrous membranes (FPU/PAN/PVB NFM) with a blocked isocyanate prepolymer (BIP) as chemical cross-linking agent were fabricated. The composite NFM, with robust mechanical, waterproof and breathable performance, has been prepared using an innovation strategy combining electrospinning with thermally induced physical bonding and chemical cross-linking. By systematically tuning the cross-linked temperature and time as well as the concentration of PVB and BIP, large breaking elongation (81.5%), good hydrostatic pressure (110 kPa) and modest WVTR (9.6 kg m−2 d−1) of the membranes were achieved. Meanwhile, the physically bonded structure and chemically cross-linked networks between PVB and BIP endowed the composite NFM with the robust tensile strength of 32.8 MPa, which was three times higher than that of pristine FPU/PAN membranes. Considering the excellent performance of the as-prepared membranes, this simple and intriguing approach may provide a versatile platform for exploring the applications of the bonded and cross-linked membranes in separation processes, membrane distillation, self-cleaning materials, and protective clothing.
Introduction
Waterproof and water-vapor-permeable membranes are critical functional materials that are designed to let water vapor pass, while prevent rain from penetrating the fabric to keep the body dry and warm.1,2 Their selective permeability for water vapor is not only interesting for protective clothing, but also has a wide range of applications in separation membranes and self-cleaning materials. Currently, the commercial waterproof and breathable (W & B) membranes have two types, such as hydrophilic thermoplastic polyurethane (TPU) nonporous membranes and polytetrafluoroethylene (PTFE) stretching microporous membranes.3,4 Hydrophilic TPU nonporous membranes offer a higher level of protection (60–80 kPa) but lower water vapor permeability (4 kg m−2 d−1) which cause some discomfort for the wearer. PTFE stretching membranes not only can give a relatively high hydrostatic pressure about 110 kPa, but also possess modest water vapor transmittance rate (WVTR) of 6.3 kg m−2 d−1. However, the major drawbacks of PTFE membranes are the difficulties in recycling and their high price. Actually, there is a negative relationship between the level of protection and comfort properties. These disadvantages restrict the practical application of W & B membranes. As a result, it is urgently needed to develop a new technology for alternative membranes which can offer a combination of adequate waterproof performance and comfort satisfaction, simultaneously.In recent years, electrospinning has been widely used as a new technology to prepare W & B nanofibrous membranes.5–7 Electrospun fibrous membranes possess high porosity, thin fiber diameter, small pore size and interconnected porous structure, which make them diffuse large amounts water vapor moisture and prevent the liquid penetration.8–10 Based on these characteristics, electrospun W & B nanofibrous membranes, including polyurethane (PU),11,12 polyacrylonitrile (PAN),13,14 polyvinylidene fluoride (PVdF),15 and nylon16 have been extensively investigated. Among these nanofibers, PAN nanofibers have attracted significant attention due to their smaller fiber diameter and higher porosity. Bagherzadeh et al. firstly prepared PAN electrospun W & B membranes with WVTR of 1 kg m−2 d−1 but low hydrostatic pressure of 3.45 kPa, which can't satisfy the practical protective and comfort needs of the garments.13 Furthermore, Wang et al. modified the PAN electrospun membranes by introducing hydrophobic agent waterborne fluorinated PU (WFPU) emulsion to achieve a high hydrostatic pressure (83.4 kPa) and WVTR (9.2 kg m−2 d−1).14 Nevertheless, the WFPU modified membranes with tensile strength of 12.1 MPa and breaking elongation of 25% can't withstand the large tension during the further combined process in the protective garment production. These poor mechanical properties tremendously limited the practical uses of the membranes.
Recently, many studies have attempted to improve the mechanical strength of nanofibers through blending polymers in electrospun process and thermal post-treatment.17–20 Chen et al. found that the mechanical performance of poly(vinylidene fluoride-co-hexafluoropropylene)/polyimide (PVdF-HFP/PI) nanofibrous membrane increased via a factor of 4–6 through heating.21 The mechanical property of the composite membrane was improved via fusing the PVdF-HFP component which has lower melting temperature. Mele et al. fabricated thermally treated electrospun networks with unique characteristics of low water contact angle hysteresis, mechanical robustness can find application in a wide ranges.22 Therefore, thermal process combined with introducing a component polymer can largely improve the mechanical property of the composite membranes.23–25 However, to our best knowledge, few efforts have been devoted to fabricating the W & B electrospun membranes utilizing the thermal treatment.
In this study, our interests focused on enhancing the mechanical and W & B performance of the electrospun membranes by introducing inter-fiber fusions and chemical cross-links through thermal treatment afterwards (as illustrated in Fig. 1a). Herein, we fabricated fluorinated polyurethane (FPU)/PAN/polyvinyl butyral (PVB)/blocked isocyanate prepolymer (BIP) composite nanofibrous membranes. The fluorination of the membranes was realized by introducing FPU component endowing the composite membranes with hydrophobic surface.26,27 The FPU modified PAN nanofibrous membranes (FPAN NFM) were acted as host polymer membranes with small fiber diameter, hydrophobic surface and high porosity. PVB possessing low softening temperature can serve as bonding fiber and BIP as the chemical cross-linking agent can occur in situ polyurethane reaction to form chemically cross-linked networks,28 which was shown in Fig. 1b. And it was identified by FT-IR spectral analysis (Fig. 2a). The key to our design is that thermal treatment impart the membranes with physically bonded nanofibrous structures and chemically cross-linked networks, which can decrease the maximum pore size (λmax) and improve the mechanical properties of the FPAN/PVB/BIP NFM. This novel non-toxic composite material may offer a new avenue for the development of a robust W & B nanofibrous membranes that meet the needs for wide application, such as protective clothing, separation process, membrane distillation, and self-cleaning materials.
 | ||
| Fig. 1 (a) Schematic showing the preparation of thermally induced cross-linked FPAN/PVB/BIP composite membranes. (b) Thermally induced chemical cross-linking of PVB and BIP. | ||
 | ||
| Fig. 2 (a) FT-IR spectrum of the pristine BIP, PVB and cross-linked PVB/BIP nanofibrous membranes. (b) The influence of heating temperature and time on tensile strength of FPAN/PVB-50/BIP-10 NFM. | ||
Experimental
Materials and reagents
PAN (Mw = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained from Kaneka Co., Ltd., Japan. PVB (Mw = 170
000) was obtained from Kaneka Co., Ltd., Japan. PVB (Mw = 170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–250
000–250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), BIP, 4,4′-diphenylmethane diisocyanate (MDI), triethylene glycol (TEG), polytetrahydrofuran (PTMEG, Mw = 1000) were provided by Aladdin Co., Ltd., China. 2-(Perfluorooctyl)ethyl alcohol (TEOH-8) was purchased from Fuxin Hengtong Fluorine Chemicals Co. Ltd., China. N,N-Dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), methanol and anhydrous calcium chloride (CaCl2) were obtained from Shanghai Lingfeng Chemical Reagent Co., Ltd., China. All these commercial products were used as received without any further treatment.
000), BIP, 4,4′-diphenylmethane diisocyanate (MDI), triethylene glycol (TEG), polytetrahydrofuran (PTMEG, Mw = 1000) were provided by Aladdin Co., Ltd., China. 2-(Perfluorooctyl)ethyl alcohol (TEOH-8) was purchased from Fuxin Hengtong Fluorine Chemicals Co. Ltd., China. N,N-Dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), methanol and anhydrous calcium chloride (CaCl2) were obtained from Shanghai Lingfeng Chemical Reagent Co., Ltd., China. All these commercial products were used as received without any further treatment.
Synthesis of FPU
The overall synthesis route of FPU was carried out according to our previous literature.29 In a 250 mL four-necked round-bottomed flask, MDI (0.05 mol) and TEOH-8 (0.21 mol) were dissolved in DMF under a N2 atmosphere with stirring at 55 °C for 1 h. Then anhydrous PTMEG (0.015 mol) as soft segment and TEG (0.02 mol) as chain extender were added to the flask, and the reactions were carried out at 65 °C for 1 h. Subsequently, TEOH-8 (0.01 mol) as the blocking agent was dissolved in DMF, transferred into a pressure-equalizing funnel and added to the flask drop wise. After complete addition, the reaction mixture was stirred for 1 h at 70 °C. After reaction, the products were washed with methanol, then deionized water and at last dried in a vacuum oven at 60 °C. The 1H and 19F nuclear magnetic resonance (NMR) spectroscopy of FPU were recorded on a Bruker Advance 400, as displayed in Fig. S1 and S2.†Preparation of FPAN/PVB/BIP NFM
PAN and FPU were dissolved in NMP to make 10 wt% solution, and the solid weight ratio of FPU was fixed to 10 wt%. A series of viscous NMP solutions containing BIP (BIP with different concentration respect to PVB: 0, 10, 20, 30, and 40 wt%) and FPAN/PVB were prepared by electrospinning, the concentration of PVB with respect to PAN in precursor solution was adjusted to 0, 25, 50, and 75 wt%. The detailed compositions and the properties of relevant solutions were listed in Table S1.† The above mentioned solutions were pumped through a capillary connected with a metal syringe needle at a constant feed rate of 1 mL h−1 by using a DXES-3 spinning equipment (Shanghai Oriental Flying Nanotechnology Co., Ltd., China). A high voltage of 25 kV was applied to the needle tip and the distance between the spinneret and a slick paper-covered grounded rotating collector was set at 20 cm. The electrospinning process was carried out at room temperature of 25 ± 2 °C and relative humidity of 45 ± 2%. All the membranes were fabricated with a thickness of 30 ± 2 μm.The chemical cross-linking of FPAN/PVB/BIP nanofibers was achieved via thermal treatment. The NFM were cut into a rectangular shape with dimensions of 160 × 160 mm each.
The membranes were placed in a vacuum oven without peeling off from the slick paper in order to prevent the shrinkage of the membranes. In the thermally induced chemical cross-linking studies, the effect of temperature was evaluated by fixing the time to 30 min while varying the cross-link temperatures (60, 80, 100, and 120 °C). Then the temperature was adjusted to 100 °C while varying the times (30, 60, 90, 120 min). The corresponding NFM were denoted as FPAN/PVB-x/BIP-y, where x is the concentration (x wt%) of PVB and y is the concentration (y wt%) of BIP. The morphology of membranes was characterized using a field emission scanning electron microscope (FE-SEM, S-4800, Hitachi Ltd., Japan). Apart from that, the porous size distribution of the membranes was measured by CFP-1100AI (Porous Materials Inc., USA). The porosity of all the NFM was calculated through the following equation:30
 | (1) |
Preparation of FPAN/PVB/BIP film
The FPAN/PVB/BIP flat film was prepared through a drop-casting process. The electrospun solutions were drop-casted onto a slide glass. After solvent evaporation, the slide glass was placed in a vacuum oven with 100 °C for 30 min to form FPAN/PVB/BIP casting film. Then, the resulting flat film was peeled from the slide glass.Measurements
The contact angle (θ) and contact angle hysteresis of the electrospun membranes and dry-casting films were measured through a Contact Angle Analyzer (Kino SL200B, USA). The analyzer was equipped with a microsyringe that can dispense 5 microliter volumes of water droplet. Contact angle hysteresis was carried out as the difference between the advancing and receding contact angles.31 As for each sample, θ and contact angle hysteresis were measured at least five times and the average values were recorded.The tensile properties of the membranes were performed by a universal testing machine with a 200 N load cell (XQ-1C, Shanghai New Fiber Instrument Co., Ltd., China). Rectangular specimens were cut to 30 × 3 mm and clamped at its cut ends for the test. Every sample was repeated at least three times to ensure the reproducibility. Since FPAN/PVB/BIP NFM were considered as candidate materials for W & B applications, a good planar tensile strength must be guaranteed using bursting test. The bursting test determining how much force is required to rupture the membrane was performed by a materials testing machine (H10K-S, Tinnitus Olsen Co., Ltd., USA) according to ISO 3303. A circular membrane sample (60 mm diameter) was fixed on the machine, and a steel ball was perpendicularly pressed on the membrane with an advancing speed of 300 mm min−1. At least three measurements were taken and averaged for each sample.
The W & B performance of the NFM was determined by testing the hydrostatic pressure and WVTR. The hydrostatic pressure of the membranes was investigated according to AATCC 127 standard test method by using a hydrostatic pressure tester (YG812C, Nantong Hongda Experiment Instruments Co., Ltd., China). The samples were fixed under a ripstop nylon woven fabric, and the water pressure increasing rate was set at 6 kPa min−1. The water vapor permeability of the fibrous membranes was measured based on ASTM E96 desiccant method using a water vapor transmission tester. A circular cup containing 33 g of CaCl2 was placed in a chamber at 38 °C and 90% RH with a wind velocity of 1 m s−1.
The WVTR was calculated according to the following equation:32
 | (2) |
Results and discussion
Effect of heating temperature and time
The cross-linking reaction between PVB and BIP was confirmed by the FT-IR spectral analysis, as shown in Fig. 2a. The peaks at 3464 cm−1 and 1434 cm−1 ascribed to –OH stretching vibration of PVB disappear in FT-IR spectra of cross-linked PVB/BIP nanofibrous membranes.28 Moreover, the presence of band at 1299 cm−1 and 1028 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of BIP also cannot be seen in the cross-linked membranes. Therefore, it is concluded that the desired chemical cross-linking was realized via the polyurethane reaction between PVB and BIP, which was shown in Fig. 1b.
C of BIP also cannot be seen in the cross-linked membranes. Therefore, it is concluded that the desired chemical cross-linking was realized via the polyurethane reaction between PVB and BIP, which was shown in Fig. 1b.
The tensile test was carried out with different temperatures and times treating the membranes to assess what conditions gave the NFM optimized physical bonding and chemical cross-linking degree. After being heated, the FPAN/PVB/BIP NFM were imparted physically bonded structures and chemically cross-linked networks, which can affect the mechanical properties of the membranes (as shown in Fig. 2b). In the case of 60 °C and 30 min, the breaking strength of 16.7 MPa was obtained. Fixing the time was 30 min, from Fig. 2b it can be seen that the breaking strength increased to 20.5 MPa when the temperature was 100 °C, and there was no obvious increasement for the strength when gradually increasing the temperature to 120 °C. When the temperature was 100 °C, there was no significant difference of the strength with the extension of heating time. It turned out that when the temperature was below 100 °C, there were no physically bonded structures and chemically cross-linked networks to improve the mechanical properties of the membranes. Moreover, when the temperature was equal to or exceeded 100 °C, the heating time had little effect on the tensile properties. Based on the above results, we chose the heating temperature of 100 °C and time of 30 min to do the following experiments.
Effect of BIP concentration
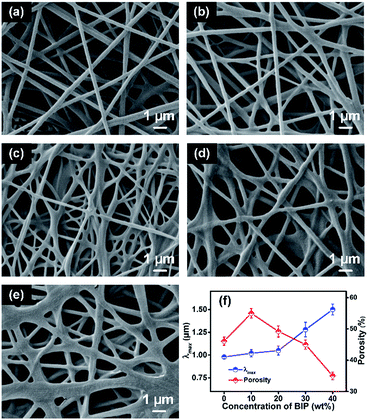
The representative FE-SEM images of FPAN/PVB-50/BIP fibrous membranes obtained by varying the concentrations of BIP (0, 10, 20, 30, and 40 wt%) with the fixed content of PVB (50 wt%) revealed a randomly oriented three dimensional nonwoven structure (Fig. 3).As shown in Fig. 3a–e, when the concentration of the BIP increased from 0 to 40 wt%, the corresponding average fiber diameters increased from 241 to 634 nm (Table S2†). These changes resulted from the obviously increased viscosity of the solution,33–35 as shown in Table S1.† More interestingly, the higher concentrations of BIP not only increased the average fiber diameter, but also induced gradually the inter-fusion of the nanofibers. In order to further demonstrate the special morphology, the porous structural characteristics of FPAN/PVB-50/BIP nanofibrous membranes were investigated (Fig. 3f and Table S2†). Based on the results, the microstructure and interconnectivity of the pores could be confirmed via the gradually decreased tortuosity caused by the decreased porosity (from 46 to 32%) and the increased pore sizes (from 0.98 to 1.50 μm) with increasing the BIP concentration.
Mechanical property of the cross-linked membranes was another key point for the practical applications of W & B membranes.36 The typical stress–strain curve (Fig. 4a) and modulus (Fig. 4b) of the NFM were measured by varying the concentration of BIP to study the chemical cross-linking degree between PVB and BIP.
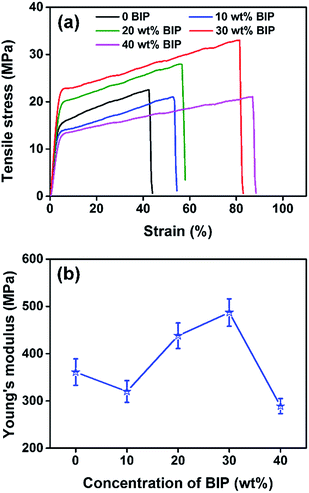 | ||
| Fig. 4 (a) Stress–strain curves and (b) Young's modulus of the thermal cross-linked fibrous membranes with various BIP concentrations. | ||
When the concentration of BIP was 10 wt%, the tensile stress of the membranes decreased to 20.5 MPa compared with 22.5 MPa of the FPAN/PVB-50/BIP-0, which resulted from that the BIP content was not enough to cross-linked with PVB. It was clearly observed that the tensile stress increased from 20.5 to 32.8 MPa and the breaking elongation increased from 52.5 to 81.5% with the concentration of BIP increasing to 30 wt%. In contrast, when the BIP content reached 40 wt%, the tensile stress decreased to 19.8 MPa and the modulus dropped due to the internal relaxed molecular structure. This was due to the solid film-like structure was too fragile to bear the tensile stress.37,38
The W & B performance of the membrane is optimized based on the following three criteria: (1) the nanofibrous membranes should have thin fiber diameter and small pore size, (2) the wettability of the membranes surface should be hydrophobic; (3) the membranes should have high porosity.39–41 The first and second requirements can satisfy the high hydrostatic pressure of the W & B membranes. In order to investigate the wetting behavior of the electrospun membranes and flat films, the θ and contact angle hysteresis values were measured, as shown in Fig. 5 and Table S3.† When increasing the BIP concentration to 20 wt%, the θ of the FPAN/PVB-50/BIP-20 nanofibrous membranes was 135° exhibiting the hydrophobic surface of the electrospun mats.42 However, when continually increasing the BIP contents gradually, the θ decreased obviously due to the declined roughness of the heated membranes.
 | ||
| Fig. 5 Contact angle of electrospun mats and dry-casting films prepared with various BIP concentrations. The inset were the photos of water drops on electrospun fiber mats and flat films surfaces. | ||
The θ of the flat films increased from 110 to 120° slightly with increasing BIP contents. The contact angle hysteresis of the dry-casting films were listed in Table S3.† The values were not changed obviously with the increasing of BIP concentration, which indicated that the flat films surfaces were fairly homogenous and there is little interaction between water and the surface. Therefore, the θ of the dry-casting films was used to represent the surface properties of the inner pores and excluded the effect of macro-sized surface morphology of the membranes, which could contribute to the resistance to liquid water penetration.
The waterproofness and breathability of the resulting nanofibrous membranes were evaluated by the hydrostatic pressure and WVTR (as shown in Fig. 6). As demonstrated in Fig. 6a, the hydrostatic pressure increased from 98.9 to 110 kPa with increasing the concentration of BIP from 0 to 30 wt%. This was the result of the increased λmax from 0.98 to 1.28 μm (as shown in Fig. 3f) and the slightly increased θ of the relevant dry-casting films (as shown in Fig. 5). However, when the concentration of BIP increased to 40 wt%, the pore size was 1.50 μm, which led to the decreasing hydrostatic pressure. In other words, the relationship among hydrostatic pressure, θ and λmax were finely accordance with the Young–Laplace equation:12,32
 | (3) |
Based on the results from Fig. 4 and 6, the FPAN/PVB-50/BIP-30 was chosen for comparison in further experiments due to its robust tensile stress (32.8 MPa), good hydrostatic pressure (110 kPa) and modest WVTR (9.6 kg m−2 d−1).
Effect of PVB concentration
In order to further investigate the evolution of the physically bonded structure of the FPAN/PVB/BIP-30 NMF (as shown in Fig. 7A), the concentrations of PVB were changed while fixing the BIP content (with respect to PVB) at 30 wt%. The FPAN/PVB/BIP-30 NMF exhibited increased average nanofiber diameter of 387, 408, 419 and 601 nm, respectively (Table S2†). As for the pure FPAN NMF, the nanofibers were observed to be loosely entangled with each other to form a randomly oriented three dimensional nonwoven structure (Fig. 7A(a)). From the Fig. 7A(b), it was obviously observed FPAN/PVB-25/BIP-30 nanofibers started to fuse together at their cross-points and came into being some bonding points, while the nanofibrous network was similar to the pristine FPAN NMF. With increasing PVB content to 75 wt%, the averaged diameters of the nanofibers increased (Table S2†) and the fusion between nanofibers were enhanced to form some bonding surface (as shown Fig. 7A(c)). Fig. 7B exhibited the corresponding schematic illustration of physically bonded structural transitions of the nanofibrous membranes.43In general, the mechanical properties of electrospun fibrous membranes were closely related to the geometric arrangement and the interfusion among the nanofibers.44,45 Fig. 7C illustrated the enlarged structural transitions of FPAN/PVB/BIP-30 NFM during the stretching of tensile test. In the case of the pure FPAN NFM, the nanofibers interpenetrated with each other and formed non-bonding structure. When the tensile stress was applied to the membranes, the nanofibers slipped through frictional entanglement leading to the stress increasing to a lower strength (8.8 MPa), as shown in Fig. 8a.
Fig. 8 clearly exhibited the enhanced tensile and bursting performance of the FPAN/PVB/BIP-30 NFM with increasing PVB concentration. The improvement in tensile strength (from 8.9 to 36.6 MPa) and bursting strength (from 8.9 to 29.7 N) were the result of the increased fiber diameter, the formation of physically bonded structure, and the chemically cross-linked networks. Firstly, the increasing in fiber diameter can help the membranes bear more load, leading to increased membrane tensile strength and bursting strength. Secondly, the enhanced physically bonded structure makes the movement of the nanofibers more difficult, thereby making the fibrous membranes more rigid. Thirdly, the thermally induced chemically cross-linked networks via the polyurethane reaction between the hydroxyl of PVB and isocyanate groups of BIP also contributed to the breaking elongation increasing from 51.9 to 125.5%.
The W & B performance of the FPAN/PVB/BIP-30 nanofibrous membranes with different PVB concentration were shown in Fig. 9. The hydrostatic pressure increased from 62.3 to 127.3 kPa, indicating that the waterproof properties increased regularly towards elevating the concentration of PVB, which was due to the declined pore size from 1.69 to 1.06 μm (Table S2†). With increasing the PVB concentration, the nanofibers got more and more inter-fusion.
As shown in Fig. 9a, the WVTR decreased from 10.5 to 6.9 kg m−2 d−1 with the decreased porosity from 85 to 30% (Table S2†). Based on the aforementioned results, it was clear to see that the hydrostatic pressure and WVTR could be regulated simultaneously by the concentration of PVB, which provide a facile and efficient method to produce functional membranes with various applications. In addition, the FPAN/PVB-50/PVB-30 fibrous membrane showed the best properties.
Synthetical evaluation of tensile strength and W & B performance
The comprehensive comparisons in Fig. 10 revealed that the electrospun fibrous membranes possessed robust W & B and mechanical properties compared with the commercial membranes (PTFE microporous and TPU hydrophilic nonporous membranes).1,2,12,28 Owing to the elastomeric performance of PU and the nonporous structure of TPU hydrophilic membranes, the TPU membranes offer the modest tensile strength (14.4–16.2 MPa) and higher level of hydrostatic pressure. However, the water vapor permeability of TPU membranes was not very good (4–5 kg m−2 d−1) due to their hydrophilic behavior. The tensile strength (9.8 MPa) of PTFE stretching membranes was lower than others due to their preparation procedures, though they possess higher hydrostatic pressure and modest WVTR (6.3 kg m−2 d−1). | ||
| Fig. 10 Comparison of tensile strength, hydrostatic pressure and WVTR among conventional W & B membranes and thermally induced cross-linked FPAN/PVB/BIP NFM. | ||
Moreover, taking advantage of the unique properties, such as thin fiber diameter, small pore size, and high porosity, the electrospun W & B membranes exhibited higher WVTR (9.2–10.9 kg m−2 d−1) and modest hydrostatic pressure (83.4–108 kPa). Nevertheless, the practical use of electrospun nanofibrous membranes was restricted by their insufficient mechanical properties (12.5–18.1 MPa). In this work, we fabricated a series of cross-linked FPAN/PVB/BIP membranes via the polyurethane reaction between PVB and BIP, which exhibited robust tensile strength (32.8–35.8 MPa) compared with the previously reported electrospun W & B membranes. Meanwhile, thanks to the physically bonded structure and chemically cross-linked networks, the FPAN/PVB/BIP NFM also exhibited higher waterproofness (110–127 kPa) and modest water moisture breathability (6.9–9.6 kg m−2 d−1), suggesting a promising candidate for a variety of potential applications in separation process, membrane distillation, self-cleaning materials and protective clothing.
Conclusions
In summary, the resulting nanofibrous composite membranes as candidate for W & B application were fabricated via electrospinning and then modified by thermally induced chemical cross-linking treatment. The mechanical and waterproof properties of the nanofibrous membranes were improved by physically bonded structures and chemically cross-linked networks between PVB and BIP. The performance of the nanofibrous membranes were systematically optimized by tuning the cross-linked temperature and time as well as the concentration of PVB and BIP. Compared to the pristine FPAN NFM, the FPAN/PVB-50/BIP-30 membranes has higher strength (32.8 MPa), larger breaking elongation (81.5%), good hydrostatic pressure (110 kPa) and modest WVTR (9.6 kg m−2 d−1). This study can provide a versatile strategy for modifying and controlling the electrospun membrane properties in W & B applications. Utilizing the thermally induced cross-linking between PVB and BIP can offer significant mechanical benefits for preparing high performance W & B membranes.Acknowledgements
This work is supported by the National Basic Research Program of China (973 Program, 2012CB525005), the National Natural Science Foundation of China (No. 51473030 and 51322304), the Fundamental Research Funds for the Central Universities, and the “DHU Distinguished Young Professor Program”.Notes and references
- A. Mukhopadhyay and V. K. Midha, J. Ind. Text., 2008, 37, 225 CrossRef CAS.
- A. Mukhopadhyay and V. K. Midha, J. Ind. Text., 2008, 38, 17 CrossRef CAS.
- S. Tabatabaei, P. Carreau and A. Ajji, J. Membr. Sci., 2008, 325, 772 CrossRef CAS.
- J. H. Pan, H. Dou, Z. Xiong, C. Xu, J. Ma and X. S. Zhao, J. Mater. Chem., 2010, 20, 4512 RSC.
- Y. K. Kang, C. H. Park, J. Kim and T. J. Kang, Fibers Polym., 2007, 8, 564 CrossRef CAS.
- R. Sahay, P. S. Kumar, R. Sridhar, J. Sundaramurthy, J. Venugopal, S. G. Mhaisalkar and S. Ramakrishna, J. Mater. Chem., 2012, 22, 12953 RSC.
- B. Ding, M. R. Wang, X. F. Wang, J. Y. Yu and G. Sun, Mater. Today, 2010, 13, 16 CrossRef CAS.
- Y. S. Park, J. W. Lee, Y. S. Nam and W. H. Park, J. Appl. Polym. Sci., 2015, 132, 41515 CrossRef.
- X. F. Wang, B. Ding, G. Sun, M. R. Wang and J. Y. Yu, Prog. Mater. Sci., 2013, 58, 1173 CrossRef CAS.
- K. A. Hong, H. S. Yoo and E. Kim, Text. Res. J., 2014, 85, 160 CrossRef.
- J. F. Ge, Y. Si, F. Fu, J. L. Wang, J. M. Yang, L. X. Cui, B. Ding, J. Yu and G. Sun, RSC Adv., 2013, 3, 2248 RSC.
- Y. Li, Z. G. Zhu, J. Y. Yu and B. Ding, ACS Appl. Mater. Interfaces, 2015, 7, 13538 CAS.
- R. Bagherzadeh, M. Latifi, S. S. Najar, M. A. Tehran, M. Gorji and L. Kong, Text. Res. J., 2011, 82, 70 CrossRef.
- J. Q. Wang, Y. Li, H. Y. Tian, J. L. Sheng, J. Y. Yu and B. Ding, RSC Adv., 2014, 4, 61068 RSC.
- F. L. Zhu, Q. Xin, Q. Q. Feng, Y. Zhou and R. T. Liu, J. Appl. Polym. Sci., 2015, 132, 42170 Search PubMed.
- K. H. Hong and T. J. Kang, J. Appl. Polym. Sci., 2006, 100, 167 CrossRef CAS.
- T. H. Cho, M. Tanaka, H. Onishi, Y. Kondo, T. Nakamura, H. Yamazaki, S. Tanase and T. Sakai, J. Power Sources, 2008, 181, 155 CrossRef CAS.
- Y. Liang, S. Cheng, J. Zhao, C. Zhang, S. Sun, N. Zhou, Y. Qiu and X. Zhang, J. Power Sources, 2013, 240, 204 CrossRef CAS.
- J. Y. Lin, X. F. Wang, B. Ding, J. Y. Yu, G. Sun and M. R. Wang, Crit. Rev. Solid State Mater. Sci., 2012, 37, 94 CrossRef CAS.
- F. E. Ahmed, B. S. Lalia and R. Hashaikeh, Desalination, 2015, 356, 15 CrossRef CAS.
- W. Chen, Y. Liu, Y. Ma, J. Liu and X. Liu, Mater. Lett., 2014, 133, 67 CrossRef CAS.
- E. Mele, J. A. Heredia-Guerrero, I. S. Bayel, G. Ciofani, G. G. Genchi, L. Ceseracciu, A. Davis, E. L. Papadopoulou, M. J. Barthel, L. Marini, R. Ruffilli and A. Athanassiou, Sci. Rep., 2015, 5, 14019 CrossRef PubMed.
- X. Liu, W. Xu, W. Li, Y. Chen and J. Rao, Polym. Eng. Sci., 2010, 50, 2400 CAS.
- Y. Si, Q. X. Fu, X. Q. Wang, J. Zhu, J. Y. Yu, G. Sun and B. Ding, ACS Nano, 2015, 9, 3791 CrossRef CAS PubMed.
- Y. Si, J. Y. Yu, X. M. Tang, J. L. Ge and B. Ding, Nat. Commun., 2014, 5, 5802 CrossRef PubMed.
- A. Davis, E. Mele, J. A. Heredia-Guerrero, I. S. Bayer and A. Athanassiou, J. Mater. Chem. A, 2015, 3, 23821 CAS.
- R. S. Kurusu and N. R. Demarquette, Langmuir, 2015, 31, 5495 CrossRef CAS PubMed.
- F. Lian, Y. Wen, Y. Ren and H. Guan, J. Membr. Sci., 2014, 456, 42 CrossRef CAS.
- N. Wang, Z. G. Zhu, J. L. Sheng, S. S. Al-Deyab, J. Y. Yu and B. Ding, J. Colloid Interface Sci., 2014, 428, 41 CrossRef CAS PubMed.
- Z. Wang, C. Zhao and Z. Pan, J. Colloid Interface Sci., 2015, 441, 121 CrossRef CAS PubMed.
- S. U. Patel, G. M. Manzo, S. U. Patel, P. S. Kulkarni and G. G. Chase, J. Nanotechnol., 2012, 2012, 483976 Search PubMed.
- L. W. Zhang, Y. Li, J. Y. Yu and B. Ding, RSC Adv., 2015, 5, 79807 RSC.
- S. Ramaswamy, L. I. Clarke and R. E. Gorga, Polymer, 2011, 52, 3183 CrossRef CAS.
- J. Gong, X. D. Li, B. Ding, D. R. Lee and H. Y. Kim, J. Appl. Polym. Sci., 2003, 89, 1573 CrossRef CAS.
- N. Wang, Y. J. Yang, S. S. Al-Deyab, M. El-Newehy, J. Y. Yu and B. Ding, J. Mater. Chem. A, 2015, 3, 23946 CAS.
- A. Gugliuzza and E. Drioli, J. Membr. Sci., 2013, 446, 350 CrossRef CAS.
- Y. Y. Zhai, N. Wang, X. Mao, Y. Si, J. Y. Yu, S. S. Al-Deyab, M. El-Newehy and B. Ding, J. Mater. Chem. A, 2014, 2, 14511 CAS.
- S. Homaeigohar, J. Koll, E. T. Lilleodden and M. Elbahri, Sep. Purif. Technol., 2012, 98, 456 CrossRef CAS.
- D. D. Lovingood, W. B. Salter, K. R. Griffith, K. M. Simpson, J. D. Hearn and J. R. Owens, Langmuir, 2013, 29, 15043 CrossRef CAS PubMed.
- W. L. Wu, Q. Z. Zhu, F. L. Qing and C. C. Han, Langmuir, 2009, 25, 17 CrossRef CAS PubMed.
- X. F. Wang, B. Ding, J. Y. Yu, Y. Si, S. B. Yang and G. Sun, Nanoscale, 2011, 3, 911 RSC.
- J. L. Sheng, Y. Li, X. F. Wang, Y. Si, J. Y. Yu and B. Ding, Sep. Purif. Technol., 2016, 158, 53 CrossRef CAS.
- S. Fan, Y. Zhang, H. Shao and X. Hu, Int. J. Biol. Macromol., 2013, 56, 83 CrossRef CAS PubMed.
- W. I. Baek, H. R. Pant, R. Nirmala, K. T. Nam, H. J. Oh and H. Y. Kim, Polym. Int., 2012, 61, 844 CrossRef CAS.
- J. H. Lee, U. S. Lee, K. U. Jeong, Y. A. Seo, S. J. Park and H. Y. Kim, Polym. Int., 2010, 59, 1683 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 19F NMR spectrum of FPU. Compositions and properties of various electrospinning solutions. Average fiber diameter, λmax and porosity of the fibrous membranes. Contact angle hysteresis of the relevant dry-casting films fabricated from various polymers solutions. See DOI: 10.1039/c5ra27913e |
| This journal is © The Royal Society of Chemistry 2016 |