DOI:
10.1039/C5RA27764G
(Paper)
RSC Adv., 2016,
6, 21210-21218
Fluorescence enhancement of rhodamine B as a tool for the determination of trace and ultra-trace concentrations of bismuth using dispersive liquid–liquid microextraction
Received
4th January 2016
, Accepted 16th February 2016
First published on 17th February 2016
Abstract
The present work describes a simple, sensitive, selective, and eco-friendly spectrofluorimetric method for bismuth determination in wastewaters and dyed scalp hair. The method is based upon fluorescence enhancement accompanying a direct reaction between rhodamine B (RB) and bismuth(III) ions, and the formed complex was then extracted from aqueous medium into a chloroform phase using dispersive liquid–liquid microextraction (DLLME). The proposed method was capable of bismuth determination even in the presence of 20 common ions in 100-fold excess over the bismuth. The plot of fluorescence enhancement measured at λex/em = 552/572 nm in the organic phase versus bismuth concentration in an aqueous solution was linear over the range of 0.5–100 μg L−1. The LOD and LOQ were found to be 0.16 and 0.532 μg L−1, respectively. The accuracy of the method was verified by analyzing bismuth in a certified standard reference material (SRM-3106). The method was also successfully applied for the analysis of bismuth in wastewaters, and dyed scalp hair samples, and the results were successfully compared with the inductively coupled plasma-mass spectrometry (ICP-MS) results. Furthermore, the structure of the newly synthesized complex was confirmed with the aid of spectroscopic measurements (UV-Vis, IR, fluorescence), and elemental and thermal analyses. These measurements indicated that the enhanced fluorescence response was attributable to coordination of Bi3+ ions with the RB reagent.
Introduction
Although the bismuth quantity in the earth's crust presents 8 μg kg−1, the chance of organisms being exposed to this element has increased due to its wide applications in medicine and industry.1,2 Bismuth citrate is used as a color additive in cosmetics intended for coloring scalp hair from grey to darker shades because of a bismuth reaction with the sulphur of hair keratin.3 According to the current US federal regulations, the use of bismuth citrate in cosmetics is subjected to the following restrictions: (i) the concentration of bismuth citrate should be less than 0.5% (w/v); and (ii) the cosmetic must be used only for coloring the scalp.3 Thus, the investigations about bismuth determination in hair have become very interest recently. The major difficulty in bismuth estimation in complex matrices, such as biological and environmental samples, is its presence at ultra-trace concentration levels. Therefore, the direct use of conventional analytical methods e.g. atomic absorption spectrometry and spectrophotometry is unsuitable for monitoring bismuth concentrations in such samples. Many spectrometric methods coupled with hydride generation techniques e.g. hydride generation-atomic fluorescence spectrometry (HGAFS),4 flow injection-hydride generation collection-atomic absorption spectrometry (FI-HGC-FAAS),5 and microwave digestion-hydride generation-atomic fluorescence spectrometry6 have been employed for the determination of bismuth at μg g−1 levels in human hair samples. However, such methods need too expensive instruments, in addition to sample introduction by hydride generation technique requires relatively large amount of sample. Thus, the determination of bismuth in small volumes of samples is challenging.7 Alternatively, low cost methods such as spectrophotometry and/or spectrofluorimetry combined with microextraction techniques have been efficiently used for metal ions determination at trace and ultra-trace concentrations and using small volumes of samples.8 Among microextraction techniques, dispersive liquid–liquid microextraction (DLLME) introduced by Assadi et al. in 2006 has found wide range of applications to the analysis of heavy metals, pesticide residues and so on9–12 due to easy to apply and excellent analytical performance compared to other microextraction techniques.
Rhodamine B; N-[9-(2-carboxyphenyl)-6-(diethylamino)-3-H xanthene-3-yliedene]-N-ethylethane ammonium chloride; with molecular formula of C28H31N2O3Cl (RB) has been widely used in analytical chemistry as an extractive reagent for the determination of metal ions by forming ion-association complexes with the general formula of [RB][MLn]. Usually, RB can exist in prototropic forms with obviously different photophysical properties, based upon its surrounding environments e.g. pH value and polarity of solvents.13 In the polar solvents, RB with carboxyl group on the phenyl ring has five isomers, lactone (R0), zwitterions (R+−) and cations (RH+, RH2++, RH3+++), equilibrating in the solution.13 The lactone form of RB with a spirocyclic conformation is colorless and nonfluorescent, while the zwitterion and univalent cation forms (R+− and RH+) with open-ring conformation are fluorescent with fluorescence quantum yield of 0.71 in ethanol. Therefore, many RB derivatives have been synthesized and employed as fluorescent ligands for the determination of some metal ions in different samples. Although, RB itself, not its derivatives, may use as monodentate ligand by carboxyl group on phenyl ring to form complexes with some metal cations,14–16 the use of such reactions is very limited in analytical chemistry until now. On the other hand, cations coordination with fluorescent ligands may cause a large increase in fluorescence intensity because the change in conformation prevents an intermolecular electron transfer responsible for fluorescence quenching due to photoinduced electron transfer.17 Therefore, any attempt to use such reactions will reduce the cost of the analysis process and improve the sensitivity of determination. Therefore, in the present work, the reaction between bismuth(III) ions and RB was carefully investigated by spectroscopic measurements and elemental and thermal analyses. Then, the fluorescence enhancement accompanying this reaction was employed in combination with DLLME to develop a spectrofluorimetric method for the determination of bismuth in human hair and water samples.
Experimental
Reagents and materials
Unless otherwise stated, all chemicals and solvents used in the present work were of analytical reagent-grade quality and were used without further purification. A stock solution of Bi(III) ions (1000 μg mL−1) was prepared from bismuth(III) nitrate (Aldrich Chemical Co Ltd., Milwaukee, WC, USA) by dissolving 0.1 g of salt in deionized water (100 mL) and in the presence of few drops of dilute HNO3 (1.0 mol L−1). More diluted standard (0.01–100 μg L−1) solutions were prepared by suitable dilution of the stock solution. A stock solution of RB (Sigma, St. Louis, MO, USA) with concentration (1 × 10−3 mol L−1) was prepared by dissolving the required weight in deionized water (100 mL). Stock solutions (1000 μg mL−1) of metal ions employed to test the selectivity were prepared from their nitrate or chloride salts in deionized water. A series of Britton–Robinson buffer, and mixture of NaOH/H2SO4 to adjust the pH of the aqueous phase in the range of 2–11 were prepared. All organic solvents used in the present work were purchased from Merck (Darmstadt, Germany).
Instrumentation
All fluorescent measurements were recorded on a Perkin-Elmer (Norwalk, CT, USA) LS 55 spectrofluorimeter, equipped with a xenon lamp and an quartz micro cell (45 mm high, internal width 4 mm and path length 10 mm) with 800 μL internal capacity. The electronic spectra in the wavelength rang of 190–1100 nm were recorded on a Perkin-Elmer (model Lambda 25, USA) spectrophotometer with 10 mm (path width) quartz cell with 4 mL internal capacity. A digital micropipette (Volac) and an Orion pH meter (model EA 940) were employed for the preparation of standard bismuth solutions and carrying out pH measurements, respectively. IR (200–4000 cm−1) spectra was recorded on Perkin Mattson 5000 FTIR spectrophotometers. A digital sensitive balance ADP 110 L with three decimal numbers was used for weighting. Rota vapor R-200 attached with Heating Bath B-490 (BUCHI) was employed for evaporating organic solvents in synthesis process of new complex. Safety-Head centrifuge (Clay Adams) with 6000 rpm has been used for collecting fine droplets. Deionized water used for the preparation of aqueous solutions was obtained from Milli-Q Plus system (Millipore, Bedford, MA, USA). A Perkin Elmer ICP-MS Sciex model Elan DRC II (California, CT, USA) was used as a reference procedure for bismuth determination at the operational parameters shown in Table 1.
Table 1 ICP-MS operational conditions for bismuth determination
| Parameter |
|
| ICP RF power (W) |
1100 |
| Nebulizer gas flow (L min−1) |
0.94 |
| Plasma gas (Ar) flow rate (L min−1) |
15 |
| Auxiliary gas (Ar) flow rate (L min−1) |
1.2 |
| Lens voltage (V) |
0.9 |
| Analog stage voltage (V) |
−1750 |
| Pulse stage voltage |
800 |
| Quadrupole rod offset std |
0 |
| Discriminator threshold |
22 |
| Cell path voltage std (V) |
−13 |
| Cell rod offset (V) |
−18 |
| Atomic mass (am) |
209 |
| Sample flow rate, mL |
93 |
Synthesis of the [Bi(C28H30N2O3)·5H2O](NO3)2 complex
In 50 mL separating funnel, 2.425 g of Bi(NO3)3·5H2O was dissolved well in deionized water after adding drops of nitric acid. Then, the obtained solution was mixed well with 2.395 g of RB reagent. The complex formed in aqueous phase was extracted by shaking twice with chloroform (2 × 10 mL) for 3 min, and the organic solvent was then evaporated gently by rotary evaporator. The residual precipitate was recrystallized from ethanol and then subjected to spectroscopic measurements and elemental, and thermal analyses. Elemental analysis of [Bi(C28H30N2O3)·5H2O](NO3)2 required 38.75% C; 4.61% H; 6.46% N; 24.1% Bi; 25.8% O. Found 38.65% C; 4.70% H; 6.53% N; 23.9% Bi; 25.2% O. The melting point of obtained complex was 153 °C.
DLLME procedure
A 5 mL of standard solutions or real samples adjusted to pH 2 by mixture of H2SO4/NaOH was mixed with RB (200 μL, 10−3 mol L−1) in an extraction vessel. A mixture of methanol (disperser solvent, 500 μL) and chloroform (extraction solvent, 150 μL) was rapidly injected into the sample tube. After manual shaking and centrifugation for 3 min at 3500 rpm, the bulk aqueous phase was removed by a syringe, and the remained organic phase was diluted to 700 μL by chloroform and then subjected to the fluorescence measurements at the excitation and emission wavelengths of 552 and 572 nm, respectively. The fluorescence enhancement of RB by Bi3+ ions added was represented by the equation:where, If and I0 are the fluorescence intensity of organic phase after and before addition of bismuth(III) ions, respectively. A blank experiment was also carried out under the same experimental conditions.
Samples preparation
Wastewaters sample. Wastewater sample collected from the wastewater treatment plant at King Abdulaziz University, Jeddah City KSA, was filtered through 0.45 μm cellulose membrane filter prior to the analysis and stored in LDPE sample bottles (250 mL) at 5 °C in the dark. Few drops of NaF (0.1% m/v) were added into sample to overcome the possible interferences of Mg2+ ions prior to the application of recommended procedure.
Hair samples. Hair samples were collected from the scalp of healthy people in the age range of 40–50 years. All hair samples were washed by water, and dried at 110 °C overnight. 0.25 g of hair dried was weighed accurately, and digested by 30.0 mL of a mixture of HClO4 and HNO3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 v/v) at 150 °C. Then, the obtained solution was evaporated until dryness, and residual solids were dissolved by drops of H2SO4 (1
8 v/v) at 150 °C. Then, the obtained solution was evaporated until dryness, and residual solids were dissolved by drops of H2SO4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The solution was transferred to a 10 mL measuring flask, and diluted to the mark with deionized water.18 An aliquot of solution was selected for applying recommended DLLME procedure after addition of drops of masking agent (NaF, 0.1% m/v).
1 v/v). The solution was transferred to a 10 mL measuring flask, and diluted to the mark with deionized water.18 An aliquot of solution was selected for applying recommended DLLME procedure after addition of drops of masking agent (NaF, 0.1% m/v).
Results and discussion
Once mixing the Bi3+ ions with RB in aqueous H2SO4 solution and shaking with chloroform, pink-colored product was formed immediately in organic phase as shown in Fig. 1(i). The electronic spectrum of RB recorded in CHCl3 phase showed one weak absorption peak at 552 ± 3 nm (Fig. 1(ii)a), however, this peak increased dramatically after mixing the Bi3+ ions with RB (Fig. 1(ii)b), this behavior is most likely attributed to the increase of RB solubility in the organic layer after the formation of the new product. Therefore, solid bismuth complex was prepared and isolated as described in the Experimental section and then subjected to spectroscopic studies, conductivity measurement, and elemental and thermal analyses.
 |
| | Fig. 1 (i) The photograph of RB in CHCl3 phase in the absence of Bi3+ ions (A), and after adding 10 μg mL−1 of Bi3+ ions (B). (ii) The electronic spectra of RB in CHCl3 phase in the absence of Bi3+ ions (a), and after shaking 10 μg mL−1 of Bi3+ ions with RB (b). | |
Structure of [Bi(RB)(H2O)5](NO3)2
The characteristic vibrational frequencies and their tentative assignments for the RB and its complex with bismuth(III) are listed in Table 2. The shift of the stretching frequencies of the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and ν(C–O) groups in the Bi(III) complex spectrum to lower frequencies at 1591 and 1271 cm−1, respectively, compared to the free RB where their frequencies appeared at 1629, and 1300 cm−1, respectively, may be due to the displacement of hydrogen ion in –COOH group with Bi3+ ion to form O–Bi bond. Another indicator of O–Bi bond formation is absence of ν(hydrogen bonding) at 2322 cm−1 in complex spectrum as shown in Fig. 2. On the other hand, NO3− ion behaves as free ionic group and does not coordinate with the Bi3+ ions where its vibrational frequency appeared at 1766 cm−1 in IR spectrum of bismuth complex conforming the presence of nitrate ions in ionic form.19 The presence of weak and broad band at 3291 cm−1 in the spectrum of the complex may be due to water molecules associated to the complex which is also confirmed by the elemental analysis and TGA data. The absence of the OH signal in RB spectrum is most likely attributed to strong intermolecular hydrogen bonding.19 Thus, the IR data may indicate that the fluorescence enhancement observed when mixing bismuth(III) ions with RB reagent is most likely attributed to the coordination of Bi3+ ions with RB by –COOH group.
O), and ν(C–O) groups in the Bi(III) complex spectrum to lower frequencies at 1591 and 1271 cm−1, respectively, compared to the free RB where their frequencies appeared at 1629, and 1300 cm−1, respectively, may be due to the displacement of hydrogen ion in –COOH group with Bi3+ ion to form O–Bi bond. Another indicator of O–Bi bond formation is absence of ν(hydrogen bonding) at 2322 cm−1 in complex spectrum as shown in Fig. 2. On the other hand, NO3− ion behaves as free ionic group and does not coordinate with the Bi3+ ions where its vibrational frequency appeared at 1766 cm−1 in IR spectrum of bismuth complex conforming the presence of nitrate ions in ionic form.19 The presence of weak and broad band at 3291 cm−1 in the spectrum of the complex may be due to water molecules associated to the complex which is also confirmed by the elemental analysis and TGA data. The absence of the OH signal in RB spectrum is most likely attributed to strong intermolecular hydrogen bonding.19 Thus, the IR data may indicate that the fluorescence enhancement observed when mixing bismuth(III) ions with RB reagent is most likely attributed to the coordination of Bi3+ ions with RB by –COOH group.
Table 2 Characteristic infrared frequencies (cm−1) of RB reagent and the complex of [Bi(RB)(H2O)5](NO3)2a
| Compound |
ν(OH) |
ν(CH)aliph |
ν(CH)arom |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
ν(C–O) |
ν(NO3−) |
ν(hydrogen bonding) |
| s = strong; w = weak. |
| RB |
— |
2848 (w) |
3103 (w) |
1629 |
1300 (S) |
— |
2322 |
| [Bi(RB)(H2O)5](NO3)2 |
3291 (w) |
2880 (w) |
2978 (w) |
1591 (S) |
1271 (S) |
1766 (S) |
— |
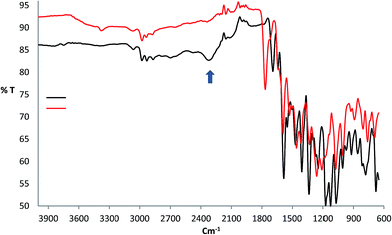 |
| | Fig. 2 FT-IR spectra of RB reagent (black) and the complex of [Bi(RB)(H2O)5](NO3)2 (red). | |
The TGA/DTA curves of bismuth complex illustrated in Fig. 3 showed two main stages, the first stage observed at 150–220 °C is due to the loss of five coordinated water molecules, (weight loss; calcd/found%; 10.52/11.2%, endothermic). The second stage was observed at 220–490 °C which is due to loss the ligand (RB) moiety and N2O3 molecule (weight loss; calcd/found%; 63.26/61.5%, exothermic). The residue due to the decomposition of the rest part of the complex was 1/2Bi2O3 (weight loss; calcd/found%; 26.22/25.02%).
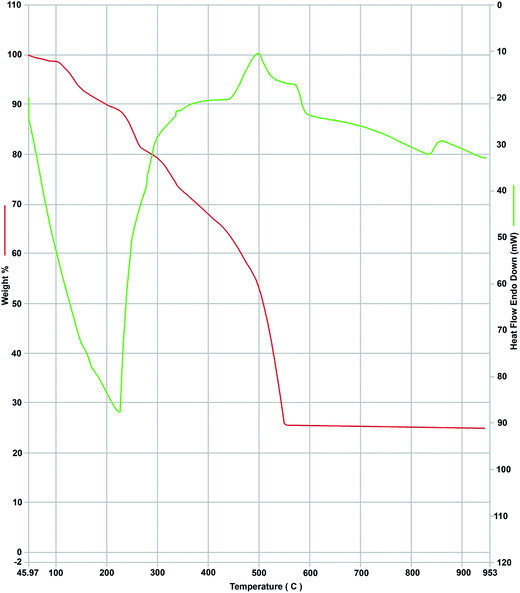 |
| | Fig. 3 TGA (red) and DTA (green) curves of the complex of [Bi(RB)(H2O)5](NO3)2. | |
Electronic spectrum (UV-Visible) of free RB recorded in CHCl3 phase revealed very weak peak at 552 ± 3 nm corresponding to the π → π* transitions. However, this peak dramatically enhanced after reaction of bismuth(III) ions with RB as demonstrated in Fig. 1(ii)b. This behavior is most likely attributed to forming new compound with higher solubility in CHCl3 phase than free RB. On the other hand, the free RB has weak fluorescence peak at λex/em = 552/569 nm in chloroform phase (Fig. 4A). However, on shaking a dilute aqueous H2SO4 solution containing RB and bismuth(III) ions with chloroform, the fluorescence intensity of RB was enhanced with a negligible shift in the maximum emission wavelength (λex/em = 552/572 nm) as demonstrated in (Fig. 4B) providing an additional indication for the formation of the complex in the ground electronic state.20 The composition of the complex formed in CHCl3 layer was determined by both of Job's continuous variation (Fig. 5) and molar ratio (not shown) methods at λmax 552 nm.21 The results revealed that, the molar ratio of RB to Bi3+ ions was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Moreover, the conditional formation constant of the bismuth complex with RB was calculated using Harvey and Manning equation:
1. Moreover, the conditional formation constant of the bismuth complex with RB was calculated using Harvey and Manning equation:
| |
 | (2) |
where
Kn is the conditional formation constant,
CR is the RB concentration,
A is the absorbance of
CR in the absence of Bi
3+ ions,
Am is the maximum absorbance, and
n is the stoichiometric obtained from Job's continuous variation and molar ratio methods, and the value of
Kn was found to be 1.8 ± 0.05 × 10
5. The value of the molar conductivity of bismuth complex was 135 ohm
−1 cm
2 mol
−1 indicating to the 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
2 electrolytic nature of the formed complex.
22 The decrease of melting point from 199 °C (free RB) to 153 °C (Bi(
III)–RB complex) may confirm the disappearance of intermolecular hydrogen bonding between RB molecules after formation of O–Bi bond.
 |
| | Fig. 4 Fluorescence spectra of RB (A), and [Bi(RB)(H2O)5](NO3)2 complex (B) in chloroform phase after applying DLLME, concentrations: RB (0.2 mL, 10−3 mol L−1), Bi3+ (10 μg L−1), conditions of DLLME are mentioned in the text. | |
 |
| | Fig. 5 Continuous variations plot for the determination of the stoichiometry of RB–Bi(III) complex. | |
Based on the results obtained from the spectroscopic measurements (IR, UV-Vis, and fluorescence), molar conductivity value, elemental analysis and TGA/DTA data, RB reacted with Bi3+ ions as monodentate ligand, therefore, the most likely composition of obtained complex is [Bi(C28H30N2O3)·5H2O](NO3)2, whereas Fig. 6 shows the possible chemical structure of this complex.
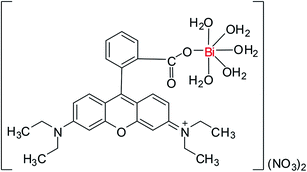 |
| | Fig. 6 The proposed chemical structure of [Bi(RB)(H2O)5](NO3)2 complex. | |
Optimization of DLLME
Effect of pH. The pH of aqueous phase is one of the most important factors affecting the efficiency of DLLME. In order to test the effect of pH on the fluorescence enhancement, the fluorescence of the organic phase before and after addition of Bi3+ ions was measured using Britton–Robinson buffer (2–11), and mixture of H2SO4/NaOH. As demonstrated in Fig. 7a, the maximum fluorescence enhancement was achieved over the pH range of 2–4. Actually, in the pH range of 2–4, the RB reagent is in the anionic form of carboxylic group since the pKa of this group is located in this pH range.23 Therefore, carboxylic group is ready to react with Bi3+ ions. While, in more acidic media (pH < 2), the formation of the complex was incomplete due to competition reactions of hydrogen ions with Bi3+ ions to react with carboxylic group of RB reagent. On the other hand, in alkaline media (pH > 6), the extraction efficiency was low owing to precipitation of bismuth hydroxide. Moreover, the fluorescence signal was more stable when using mixture of H2SO4/NaOH compared to Britton–Robinson buffer. Therefore, the pH of 2 adjusted by mixture of H2SO4/NaOH was selected as the optimum value for subsequent work.
 |
| | Fig. 7 Effect of various parameters on the DLLME of Bi. (a) pH, (b) the extractant type, (c) the disperser solvent type, and (d) RB concentration. | |
Effect of type and volume of extractant. For obtaining high efficiency of DLLME, careful attention should be paid to the selection of the type and volume of the extractant. The solvents used in normal DLLME as extraction solvent should have lower solubility in water and high ability of extraction of analyte. Thus, dichloromethane, chloroform and carbon tetrachloride were investigated as extractant with methanol as disperser solvent. As shown in Fig. 7b, higher signal of fluorescence was observed in the case of using chloroform. Moreover, different volumes of chloroform were employed with 500 μL methanol as disperser solvent for extraction of 100 μL L−1 bismuth(III) ions. The fluorescence intensity increased with increasing the volume of CHCl3 to 150 μL and then remained approximately constant by further increases. Therefore, 150 μL was used in recommended procedure.
Effect of type and volume of disperser solvent. The fact is that disperser solvent plays an important role in dispersion, stability and equilibrium of the ternary-phase system in DLLME. Thus, the selection of disperser solvent is critical and largely affects the efficiency of extraction and the enhancement factor. The solvent used as disperser should be miscible with the aqueous sample and extraction solvent, thus, proper solvents including acetone, methanol, ethanol and acetonitrile were studied to select the best disperser solvent for the proposed method. The results shown in Fig. 7c, indicated that methanol was the best disperser solvent for the extraction of bismuth. Therefore, the effect of its volume on the extraction efficiency was tested using series of different volumes (300–1500 μL) in the presence of 150 μL chloroform. The results showed that in the case of 500 μL, the highest fluorescence enhancement was attainable.
Effect of RB concentration. The variation of the fluorescence enhancement as a function of the RB concentration was evaluated by increasing volumes of the reagent (10−3 mol L−1) from 50 to 350 μL at the optimum experimental conditions. The results shown in Fig. 7d, indicated that 200 μL of RB (10−3 mol L−1) was sufficient to extract bismuth(III) ion up to 100 μg L−1. Although, the solubility of RB in chloroform phase is low, it has significant fluorescence signal in this phase as shown in Fig. 4A. On the other hand, fluorescence enhancement due to the formation of new complex was noticed at a wavelength very close to that of free RB. Thus, the fluorescence signal of unreacted RB will increase with increasing the amount of free RB in CHCl3 phase to cover the fluorescence signal of complex formed in same phase. So, small excess of RB is necessary to complete the reaction of complex formation, but, very large excess, over 500 μL, causes strong overlap between fluorescence spectra of free RB and its complex with bismuth. As a result of this overlap, the fluorescence enhancement becomes indistinguishable. On the other hand, the amounts of RB which are smaller than the recommended value gave incomplete extraction. Thus, the volume of 200 μL was used in further experiments.
The effect of type and volume of dilution solvents. Some organic solvents including, ethanol, methanol, acetone, acetonitrile and chloroform were investigated as dilution solvents. However, chloroform gave the best signal compared to the other solvents. Therefore, the influence of its volume on the fluorescence enhancement was tested using volumes in the range of 700–1500 μL. The obtained results showed that a 700 μL of chloroform was the best volume to get optimized intensity of fluorescence.
Effect of centrifugation. To collect the droplets of extractant dispersed in the aqueous phase after shaking, the mixture must be centrifuged, thus, the speed and time of centrifugation were investigated in the range of 2000 to 5000 rpm and 1–5 min, respectively. The droplets of extraction solvent were completely collected and sedimented to the bottom of tube after using centrifugation with speed 3500 rpm for 3 min.
Effect of extraction time. Extraction time is one of the most key factors in the most of extraction procedures. In DLLME, extraction time is defined as the time between the injection of mixture of disperser solvent and extractant and beginning centrifugation. The influence of extraction time was examined in the range of less than 1 to 15 min. The results showed that the extraction accomplished in a very short time after the formation of cloudy solution and the equilibrium state was achieved quickly, which was one of the main advantages of DLLME. The fact is that, the complex formed between bismuth and reagent diffuses quickly into the extraction solvent due to the infinitely large surface area between extraction solvent and aqueous phase after the formation of cloudy solution. Thus, the extraction time of 1 min was chosen as the optimum condition for the proposed method. Actually, the only time-consuming step, that required about 3 min, was centrifugation performed for collecting the fine droplets of extractant dispersed in aqueous phase.
Selectivity. The selectivity of the developed method was examined in the presence of various cations and anions under the established conditions. The tolerance limits of the coexisting ions, defined as the largest amount of foreign ion that causes an error in the bismuth determination larger than ±5%, are given in Table 3. The 20 common ions did not interfere even when they are present in 100-fold excess over the bismuth. Only Mg2+, Cd2+ interfered seriously with bismuth determination. However, the interference of Mg2+ was eliminated by adding few drops of NaF (0.1% m/v). Hence, the method is adequately selective for the bismuth determination in water and other matrices.
Table 3 Tolerance limits of interfering species in bismuth(III) (50.0 μg L−1) determination by the developed method
| Interfering species |
Interfering to analyte ratio |
| Li+, Na+, K+, Ag+, Cl−, F−, NO3−, SO42−, CO32−, tartrate |
1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Hg2+, Mn2+, Ca2+, Ni2+, Co2+, Cu2+, CN− |
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Fe3+, Cr3+, Zn2+, Pb2+ |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Cd2+, Mg2+ |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Analytical performance of the method. Analytical figure of merit of proposed DLLME spectrofluorimetric method was obtained under the optimized experimental conditions and summarized in Table 4. The plot of bismuth(III) concentration vs. fluorescence enhancement (ΔI) was linear in the range of 0.5–100 μg L−1, and the following linear regression equation was achieved:| | |
ΔI = 2.97C − 0.05023 (r2 = 0.992, n = 6)
| (3) |
where, ΔI is the fluorescence enhancement after applying DLLME; C is the initial concentration of bismuth in μg L−1. The lower limit of detection (LOD) and quantification (LOQ) of the developed method were calculated using the equationswhere, S is the standard deviation of the blank (n = 6), and b is the slope of the calibration plot.24 The results shown in Table 4 revealed that the application of DLLME has dramatically improved the analytical characteristics of the proposed procedure in terms of LOD, LOQ, and R.S.D in comparison with LLE.
Table 4 Analytical features of the developed DLLME (A) and normal LLE (B) methods
| Analytical performance |
A |
B |
| Linear range (μg L−1) |
0.5–100 |
5–200 |
| Correlation coefficient (R2) |
0.992 |
0.991 |
| Slope |
2.97 |
0.612 |
| R.S.D. (%) |
0.457 |
1.301 |
| Detection limit, LOD (μg L−1) |
0.16 |
3.541 |
| Limit of quantification, LOQ (μg L−1) |
0.532 |
10.623 |
Analytical applications
Analysis of bismuth in the standard reference material 3106. Standard reference material (SRM-3106) was purchased from National Institute of Standards & Technology and analyzed to test the accuracy of the proposed method for bismuth determination. According to manufacture, SRM-3106 contains Si and Na at concentration level of 20 mg kg−1, while the concentrations of B and La are in the range of 1–5 mg kg−1, and the concentration levels of Al, As, Ca, Ce, Mg, Mn, Rb, and Zn are in the range 0.05–1 mg kg−1. Good agreement between certified value of bismuth in SRM-3106 (10.00 ± 0.02 mg g−1) and the value obtained by the developed method (9.82 ± 0.08 mg g−1).
Analysis of bismuth in scalp hair and wastewater. The validation of the proposed method for the assay of the bismuth in scalp hair and wastewater samples was critically investigated by the direct calibration plot and the standard addition method. All samples were analyzed for bismuth determination by the developed spectrofluorimetric (A) and ICP-MS (B) methods before and after adding various known concentrations (50–100 μg L−1) of bismuth(III) ions and the results are shown in Table 5. The recovery of both methods was in good agreement and always higher than 95% confirming the accuracy of developed procedure and its independence from matrix interference. Moreover, the precision of two methods was validated by F-test for samples before addition of known concentrations of bismuth(III) ions. In all cases, the F values calculated were always less than the tabulated value at 95% confidence level as shown in Table 5. Therefore, there is no significant difference in the precision of two methods for bismuth determination in tested samples at the 95% confidence level.25
Table 5 The analysis of bismuth in scalp hair and wastewaters using the developed spectrofluorimetric (A) and ICP-MS (B) methods
| Sample |
Bismuth added, (μg L−1) |
Bismuth found, (μg L−1) |
Recovery, (%) |
F-valueb |
| A |
B |
A |
B |
| Mean value ± standard deviation (n = 10). F9,9 = 3.179 at 95% confidence level. |
| Dyed scalp hair (1) |
— |
0.612 ± 0.1a |
0.625 ± 0.25 |
— |
— |
1.29 |
| 5 |
5.8 ± 0.9 |
5.7 ± 0.8 |
103.3 |
101.1 |
| 10 |
10.2 ± 2.3 |
9.8 ± 0.7 |
96 |
92 |
| Dyed scalp hair (2) |
— |
0.778 ± 0.2 |
0.810 ± 0.08 |
— |
— |
1.18 |
| 20 |
21.2 ± 1.3 |
21.6 ± 0.9 |
102 |
104 |
| 30 |
29.3 ± 1.8 |
30.1 ± 0.8 |
95 |
97.6 |
| Wastewaters |
— |
1.5 ± 0.5 |
1.6 ± 0.9 |
— |
— |
1.09 |
| 50 |
50.3 ± 1.8 |
51.4 ± 0.8 |
97.6 |
99.6 |
| 100 |
103.4 ± 3.1 |
101.2 ± 1.4 |
103.4 |
101.2 |
Conclusion
In this study, the synthesis and characterization of the new complex of RB with bismuth(III) ions were discussed in details and the most likely composition of this complex was proposed. Based upon fluorescence enhancement associated to the formation of Bi(III)–RB complex, we developed a simple, low cost and sensitive method for preconcentration and determination of bismuth employing DLLME combined with spectrofluorometry. According to our knowledge, this is the first time in which RB reagent is directly used to determine bismuth without the need to prepare new derivatives. A comparison of the analytical features of the method submitted in the present work with previously published methods e.g. hydride generation coupled with ICP-OES,26 adsorptive stripping voltammetry,27 flow injection-potentiometry,28 spectrophotometry29–32 and fluorimetry2 is given in Table 6. From comparison, most of the reported methods suffer from many drawbacks e.g. high cost, the consumption of large amounts of toxic organic solvents, time consuming and the low sensitivity and selectivity, while the proposed method offers a simple methodology coupled with good reproducibility and accuracy, and low cost. Furthermore, the LOD is much lower than the maximum allowable level (MAL) of bismuth recommended by World Health Organization (WHO) in water and it is favorably compared with the LOD of many spectrochemical (e.g. GFAAS, FAAS and direct ICP-OES) and electrochemical techniques.
Table 6 Comparison of the procedure developed in the present study with some previously published methods for determination of bismuth
| The method |
LOD (μg L−1) |
Linear range, (μg L−1) |
Remarks |
Reference |
| Fluorescence quenching |
50 |
130–2090 |
Low sensitivity and required large volume of toxic materials |
2 |
| Hydride of bismuth-ICP-OES |
0.2 |
1–50 |
Sensitive but expensive and required large amounts of sample |
26 |
| Adsorptive stripping voltammetry |
0.17 |
0.42–42 |
Sensitive but use of hanging mercury dropping electrode |
27 |
| Flow injection-potentiometric |
2500 |
4200–2.1 × 106 |
Low sensitivity, high LOD, expensive and used toxic organic material |
28 |
| Micro extraction-spectrophotometry |
300 |
1000–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Low sensitivity |
29 |
| DLLME-spectrophotometry |
1.6 |
4–400 |
Moderate sensitivity |
30 |
| First-derivative spectrophotometry |
— |
500–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Low sensitivity and selectivity |
31 |
| β-Correction spectrophotometry |
200 |
200–3200 |
Less sensitivity and time-consuming |
32 |
| LLE-fluorescence enhancement |
3.54 |
5–200 |
Sensitive, simple, low cost but required large volume of toxic organic solvent |
Present work |
| DLLME-fluorescence enhancement |
0.16 |
0.5–100 |
Sensitive, simple, low cost, selective and environmentally friendly, the interferences of Cd2+, Mg2+ |
Present work |
References
- M. S. El-Shahawi, A. Hamza, A. A. Al-Sibaai and H. M. Al-Saidi, Chem. Eng. J., 2011, 173, 29–35 CrossRef CAS.
- M. A. Taher, M. Rahimi and H. Fazelirad, Sensitive, J. Lumin., 2014, 145, 976–980 CrossRef CAS.
- SCCS (Scientific Committee on Consumer Safety), Opinion on bismuth citrate, 12 December 2013.
- B. Liu, F. Wu, X. Li, Z. Fu, Q. Deng, C. Mo, J. Zhu, Y. Zhu and H. Liao, Microchem. J., 2011, 97, 20–24 CrossRef CAS.
- S. Y. Chen, Z. F. Zhang and H. M. Yu, Anal. Bioanal. Chem., 2002, 374, 126–130 CrossRef CAS PubMed.
- L. Rahman, W. T. Corns, D. W. Bryce and P. B. Stockwell, Talanta, 2000, 52, 833–843 CrossRef CAS PubMed.
- Y. Chen, M. Li, L. Fu, X. Hou and X. Jiang, Microchem. J., 2014, 114, 182–186 CrossRef CAS.
- M. S. El-Shahawi and H. M. Al-Saidi, TrAC, Trends Anal. Chem., 2013, 44, 12–24 CrossRef CAS.
- M. A. Farajzadeh, D. Djozan and R. F. Bakhtiyari, Talanta, 2010, 81, 1360–1367 CrossRef CAS PubMed.
- S. R. Yousefi and F. Shemirani, Anal. Chim. Acta, 2010, 669, 25–31 CrossRef CAS PubMed.
- M. Rezaee, Y. Yamini, A. Khanchi, M. Faraji and A. Saleh, J. Hazard. Mater., 2010, 178, 766–770 CrossRef CAS PubMed.
- H. M. Al-Saidi, Eur. J. Chem., 2012, 3, 202–207 CrossRef CAS.
- Y. Wan, Q. Guo, X. Wang and A. Xia, Anal. Chim. Acta, 2010, 665, 215–220 CrossRef CAS PubMed.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465–1472 RSC.
- V. Dujols, F. Ford and A. W. Czarnik, J. Am. Chem. Soc., 1997, 119, 7386–7387 CrossRef CAS.
- Q. Zhang, Q. Lin, L. F. Wang, L. J. Mu and X. Y. Huang, Pol. J. Chem., 2000, 74, 639–648 CAS.
- M. H. Keefe, K. D. Benkstein and J. T. Hupp, Coord. Chem. Rev., 2000, 205, 201 CrossRef CAS.
- H. Fazelirad and M. A. Taher, Ligandless, Talanta, 2013, 103, 375–383 CrossRef CAS PubMed.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 3rd edn, 1978 Search PubMed.
- M. S. El-Shahawi, H. M. Al-Saidi, A. S. Bashammakh, A. A. Al-Sibaai and M. A. Abdelfadeel, Talanta, 2011, 84, 175–179 CrossRef CAS PubMed.
- D. T. Sawyer, W. R. Heinemann and J. M. Beebe, Chemistry Experiments for Instrumental Methods, John Wiley& Sons, 1984 Search PubMed.
- W. J. Geary, Coord. Chem. Rev., 1971, 7, 81–122 CrossRef CAS.
- J. L. Pittman, K. F. Schrum and S. D. Gilman, Analyst, 2001, 126, 1240–1247 RSC.
- J. C. Miller and J. N. Miller, Statistics and Chemometrics for Analytical Chemistry, Pearson Education Limited, Prentice Hall, New York, 6th edn, 2010 Search PubMed.
- G. D. Christian, Analytical chemistry, John Wiley & Sons, 6th edn, 2004 Search PubMed.
- D. D. Afonso, S. Baytak and Z. Arslan, J. Anal. At. Spectrom., 2010, 25, 726 RSC.
- A. Koper and M. Grabarczyk, J. Electroanal. Chem., 2011, 663, 67–71 CrossRef CAS.
- M. F. S. Teixeira and O. Fatibello-Filho, Int. J. Pharm., 2001, 221, 115–121 CrossRef CAS PubMed.
- K. Wrobel, K. Wrobel and E. M. ColungaUrbina, Microchim. Acta, 2000, 135, 87–90 CrossRef CAS.
- S. Rastegarzadeh, N. Pourrezaa and A. Larkia, Anal. Methods, 2014, 6, 3500–3505 RSC.
- A. Afkhami and A. R. Zarei, Anal. Sci., 2003, 19, 917–921 CrossRef CAS PubMed.
- A. Abbaspour and L. Baramakeh, Talanta, 2005, 65, 692–699 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 v/v) at 150 °C. Then, the obtained solution was evaporated until dryness, and residual solids were dissolved by drops of H2SO4 (1
8 v/v) at 150 °C. Then, the obtained solution was evaporated until dryness, and residual solids were dissolved by drops of H2SO4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The solution was transferred to a 10 mL measuring flask, and diluted to the mark with deionized water.18 An aliquot of solution was selected for applying recommended DLLME procedure after addition of drops of masking agent (NaF, 0.1% m/v).
1 v/v). The solution was transferred to a 10 mL measuring flask, and diluted to the mark with deionized water.18 An aliquot of solution was selected for applying recommended DLLME procedure after addition of drops of masking agent (NaF, 0.1% m/v).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and ν(C–O) groups in the Bi(III) complex spectrum to lower frequencies at 1591 and 1271 cm−1, respectively, compared to the free RB where their frequencies appeared at 1629, and 1300 cm−1, respectively, may be due to the displacement of hydrogen ion in –COOH group with Bi3+ ion to form O–Bi bond. Another indicator of O–Bi bond formation is absence of ν(hydrogen bonding) at 2322 cm−1 in complex spectrum as shown in Fig. 2. On the other hand, NO3− ion behaves as free ionic group and does not coordinate with the Bi3+ ions where its vibrational frequency appeared at 1766 cm−1 in IR spectrum of bismuth complex conforming the presence of nitrate ions in ionic form.19 The presence of weak and broad band at 3291 cm−1 in the spectrum of the complex may be due to water molecules associated to the complex which is also confirmed by the elemental analysis and TGA data. The absence of the OH signal in RB spectrum is most likely attributed to strong intermolecular hydrogen bonding.19 Thus, the IR data may indicate that the fluorescence enhancement observed when mixing bismuth(III) ions with RB reagent is most likely attributed to the coordination of Bi3+ ions with RB by –COOH group.
O), and ν(C–O) groups in the Bi(III) complex spectrum to lower frequencies at 1591 and 1271 cm−1, respectively, compared to the free RB where their frequencies appeared at 1629, and 1300 cm−1, respectively, may be due to the displacement of hydrogen ion in –COOH group with Bi3+ ion to form O–Bi bond. Another indicator of O–Bi bond formation is absence of ν(hydrogen bonding) at 2322 cm−1 in complex spectrum as shown in Fig. 2. On the other hand, NO3− ion behaves as free ionic group and does not coordinate with the Bi3+ ions where its vibrational frequency appeared at 1766 cm−1 in IR spectrum of bismuth complex conforming the presence of nitrate ions in ionic form.19 The presence of weak and broad band at 3291 cm−1 in the spectrum of the complex may be due to water molecules associated to the complex which is also confirmed by the elemental analysis and TGA data. The absence of the OH signal in RB spectrum is most likely attributed to strong intermolecular hydrogen bonding.19 Thus, the IR data may indicate that the fluorescence enhancement observed when mixing bismuth(III) ions with RB reagent is most likely attributed to the coordination of Bi3+ ions with RB by –COOH group.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)
O)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Moreover, the conditional formation constant of the bismuth complex with RB was calculated using Harvey and Manning equation:
1. Moreover, the conditional formation constant of the bismuth complex with RB was calculated using Harvey and Manning equation:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 electrolytic nature of the formed complex.22 The decrease of melting point from 199 °C (free RB) to 153 °C (Bi(III)–RB complex) may confirm the disappearance of intermolecular hydrogen bonding between RB molecules after formation of O–Bi bond.
2 electrolytic nature of the formed complex.22 The decrease of melting point from 199 °C (free RB) to 153 °C (Bi(III)–RB complex) may confirm the disappearance of intermolecular hydrogen bonding between RB molecules after formation of O–Bi bond.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000






