Surface modification of CdSeS nanocrystals for polymer hybrid solar cells†
A. Erdogana,
C. Karakayabe,
M. K. Goncea,
S. Buyukcelebia,
E. Yenela,
K. Karaa,
A. N. Ozcivanc,
M. Cand,
M. Kus*a and
S. Demic*e
aAdvanced Technology Research and Application Center Selcuk University, 42030, Konya, Turkey. E-mail: mahmutkus1@gmail.com; Fax: +903322412143; Tel: +903322233414
bDepartment of Material Engineering, Celal Bayar University, 45040-Muradiye, Manisa, Turkey
cDepartment of Electrical and Electronics Engineering, İzmir Katip Celebi University, 35620, Cigli, Izmir, Turkey
dDepartment of Engineering Sciences, İzmir Katip Celebi University, 35620, Cigli, Izmir, Turkey
eDepartment of Materials Science and Engineering, İzmir Katip Celebi University, 35620, Cigli, Izmir, Turkey. E-mail: serafettin.demic@gmail.com
First published on 2nd March 2016
Abstract
We report the synthesis of fluorene–carbazole derivatives as capping agents for CdSeS nanocrystals and present their performance in polymer hybrid solar cells. CdSeS nanocrystals and different ligands consisting of fluorene and carbazole units were synthesized and characterized. Both oleic acid and pyridine capped CdSeS nanocrystals were used as reference materials in polymer hybrid solar cells. We observed that our synthesized materials show better efficiencies depending on their structures. In comparison with the reference cells consisting of pyridine capped CdSeS nanocrystals, ligand capped CdSeS shows better efficiency due to electron withdrawing and accepting groups in its structure. The reason behind the superiority of our ligands compared to the reference pyridine is the donor and/or acceptor based compatibility of the combined structures and effective surface modification as well.
Introduction
In hybrid solar cells, P3HT (poly(3-hexylthiophene-2,5-diyl)) polymers with a donor character are generally combined with a fullerene counterpart that has an acceptor character. Yet, Cd based quantum dots are favored in recent hybrid solar cell research as an alternative to fullerene for their acceptor character as well.1–3 There are several reasonable capabilities of Cd based particles, so they are widely preferred instead of the well-known and considerably superior fullerene acceptors.1,4–6 However, in spite of the theoretical advantage of CdSeS nanocrystal based polymer hybrid solar cells, every made polymer–CdSeS nanocrystal device demonstrated rather low performance (∼6%) as compared to fullerene based examples.1 On the contrary, bandgap and frontier energy analysis on polymers and nanocrystals has led researchers to have hopeful expectations.1,7Colloidal nanocrystals are useful during the solution stage of solar cell fabrication and can be synthesized using isolating and long-chained organic ligands.1 The reason for this is that those aliphatic ligands act as stabilizing agents during the synthesis and ensure colloidal dispersibility in nonpolar solvents. In addition, when the metal cations on the nanocrystal surfaces are coordinated, the surfaces acquire an electronic state to allow trapping with a suitable band gap.1,8–11 Therefore, such capping agents mostly prevent electron transfer from polymers to nanocrystals. So, ligand processing is especially crucial in hybrid solar cell fabrication due to the restrictions mentioned. Hence, electrically isolating native ligands should be replaced with ones that provide electron transfer from the polymer to the nanocrystal.12–16 The most well-known method to realize this is called the “ligand exchange method”.13 The most important parameter for the achievement of this method is the completeness of the exchange.1 For such an approach, the ligand exchange method using pyridine has been preferred as a proper surface modification technique for CdSe nanocrystals so far.1,12
Pyridine has been used for capping various nanocrystals and tried with different surface active agents in order to gain better hybrid cell efficiencies.17 However, the power conversion efficiencies have remained below 6% up to now.1,7,12,18 The most common reasons behind acquiring such low levels are the donor–acceptor incompatibility of the combined structures within the hybrid cell and ineffective surface modification.1,2,5,7,8,12 The key parameters for the compatibility of ligand–nanocrystal and ligand–polymer are the structural configuration and functional groups on the ligands.14 Therefore, designing new molecules as ligands is a basic need for the improvement of hybrid solar cell performance.
In this work, we designed and synthesized new ligands as capping agents for CdSeS nanocrystals and then investigated their performance in hybrid solar cells. At the first hand three different ligands consisting of only donor, donor–acceptor and only acceptor groups were synthesized. Then, these ligands were used as ligand exchange materials as well as direct capping agents in nanocrystal synthesis. We observed that ligand exchange is a better way than directly making the synthesis using these ligands. Finally, the hybrid solar cells were fabricated using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ligand capped nanocrystal to P3HT ratios. With this study, we briefly showed the importance of molecular design and the functional groups present on the ligands for the surface passivation of nanocrystals used in hybrid solar cells.
9 ligand capped nanocrystal to P3HT ratios. With this study, we briefly showed the importance of molecular design and the functional groups present on the ligands for the surface passivation of nanocrystals used in hybrid solar cells.
Experimental section
The synthesis of the ligands and CdSeS nanocrystals, the ligand exchange method, the fabrication procedure of the solar cells and all related characterization processes are detailed in the ESI† section. Fig. 1 shows the chemical structures of three ligands MC1, MC2 and L1 used in the modification of the nanocrystal surfaces.Structural characterization of the molecules was verified using 1H and 13C NMR spectra (see ESI Fig. S-1†). XRD results verify that the products (i.e. nanocrystals) are in alloy form (see ESI Fig. S-2†), where the alloy structure is clearly indicated by peaks emerging between the diffraction angle peaks of pure CdSe and CdS nanocrystals. For further characterization, an HRTEM image, UV-vis absorption spectra, fluorescence spectra and FTIR spectra are also given in the ESI (see Fig. S-3–S-6†). Electrochemical studies were carried out for the ligands (L1, MC1 and MC2 molecules) as well as the ligand capped nanocrystals. The measurements were carried out by using the well-known three-electrode system: a glassy carbon electrode (as working electrode), a platinum wire (as auxiliary electrode) and a silver wire (as reference electrode of Ag/Ag+). The molarity of the electrolyte solution (TPAPF6) inside the acetonitrile was 0.1 M.
Results and discussion
Electrochemical properties
In Fig. 2, the cyclic voltammetry (CV) measurements belonging to the pure molecules are given which give clues on the energy band compatibility of these molecules by means of HOMO and LUMO calculations.19 Energetic compatibility is particularly important and mainly determined by the functional groups present on the molecular structures.20In Fig. 2a, two reversible oxidation peaks are observed for L1 as expected where the first peak starts at 1.3 eV and reduction starts at 1.2 eV. These two oxidation peaks correspond to carbazole units. Since it has no reducible moiety in its structure, there is not any reduction signal in the cathodic region. The MC1 molecule (see Fig. 2b), having 2 cyano units and one carbazole body, shows two reversible reduction peaks from the cyano groups (at −0.3 eV and −0.5 eV) and one reversible oxidation peak (at 1.2 V) from the carbazole group. MC2 exhibits similar reduction peaks to those of the MC1 molecule (reductions at −0.7 eV and −0.8 eV) (Fig. 2c).
Cyclic voltammograms of the nanocrystals capped with L1, MC1 and MC2 ligands are given in Fig. 3a–c. CdSeS capped with L1 shows two reversible oxidation peaks and one semi-reversible reduction peak at 1.1, 1.4 and −0.7 V respectively. It is obvious that the oxidations correspond to the carbazole groups of L1. The observation of a semi-reversible reduction signal in the cathodic region of the ligand capped nanocrystal arises from the CdSeS nanocrystals.21
 | ||
| Fig. 3 CV measurement of hybrid structures that are derived by the use of the ligand exchange method, (a) L1-QD hybrid material, (b) MC1-QD, and (c) MC2-QD. | ||
The CV results belonging to the nanocrystal prepared from the MC1 molecule are given in Fig. 3b. The presence of electron donating (carbazole) and accepting (cyano and CdSeS) moieties in the MC1 ligand capped nanocrystal is clearly indicated by neat reduction and oxidation signals in the corresponding voltammetry results. When the current density and charge in the observed reduction peak are investigated, one can conclude that two cyano groups and CdSeS are simultaneously reduced. Hence, the observed reduction signal in the cathodic region (between 1.1 eV and 1.2 eV) is broader than that of the pure MC1 molecule (between −0.3 eV and −0.5 eV).
The CV results obtained from the MC2 capped nanocrystal are presented in Fig. 3c. When the molecular structures of MC1 and MC2 are compared, the carbazole unit in MC1 is replaced by fluorene in MC2. Such a replacement implies that there would be no oxidation peak in the anodic region for the MC2 capped nanocrystal. On the other hand, both hybrid structures prepared from MC1 and MC2 are expected to give reduction peaks, because of the presence of cyano groups in both molecules. It can be inferred from the cyclic voltammograms that the MC2 capped nanocrystal behaves much the same as the MC1 capped nanocrystal, because both have almost identical acceptors surrounding them. Finally all these CV observations clearly demonstrate that ligands L1, MC1 and MC2 are capped to the surface of CdSeS quantum dots to generate hybrid nanocrystals.
As an alternative to the ligand exchange method, MC1 and MC2 molecules were also processed with the direct synthesis method with CdSeS, called the surface active agent method (see Fig. 4) (XRD results are given in ESI Fig. S-7†). The hybrid nanocrystals obtained by this method were also subjected to CV investigations. The hybrid nanocrystals produced similar oxidation and reduction signals to those obtained by the ligand exchange method.
 | ||
| Fig. 4 Cyclic voltammetry measurements of hybrid nanocrystals derived by the direct synthesis method, (a) MC1-QD, and (b) MC2-QD. | ||
Solar cell performances
Hybrid solar cells were fabricated and characterized with L1, MC1 and MC2 capped nanocrystals (L1-QD, MC1-QD and MC2-QD). At the beginning, different ratios of CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT were investigated for each ligand capped nanocrystal. Since pyridine is known as the best capping agent for solar cell applications of nanocrystals, pyridine capped CdSeS
P3HT were investigated for each ligand capped nanocrystal. Since pyridine is known as the best capping agent for solar cell applications of nanocrystals, pyridine capped CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
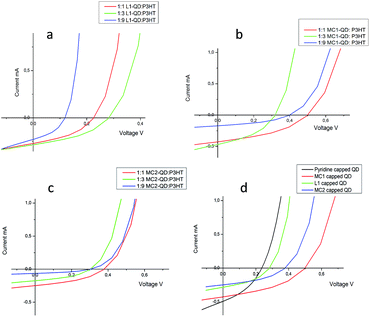
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT solar cells were used as the reference device. For each of the hybrid nanocrystals, all cells were prepared and tested under the same conditions. The active areas of the cells were 0.12 cm2. All cells were held in a glove box environment and measurements were carried out in the same conditions under an AM 1.5 solar simulator. AFM analyses of some films are given in the ESI (see Fig. S-8†). Fig. 5a–c shows I–V characteristics of L1-QD (a), MC1-QD (b) and MC2-QD (c) based solar cells with different CdSeS
P3HT solar cells were used as the reference device. For each of the hybrid nanocrystals, all cells were prepared and tested under the same conditions. The active areas of the cells were 0.12 cm2. All cells were held in a glove box environment and measurements were carried out in the same conditions under an AM 1.5 solar simulator. AFM analyses of some films are given in the ESI (see Fig. S-8†). Fig. 5a–c shows I–V characteristics of L1-QD (a), MC1-QD (b) and MC2-QD (c) based solar cells with different CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT ratios.
P3HT ratios.
The best results for L1-QD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT, MC1-QD
P3HT, MC1-QD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT and MC2-QD
P3HT and MC2-QD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT ratios were found to be 1
P3HT ratios were found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively (see Fig. 5a–c). The optimization of the pyridine capped CdSeS
1 respectively (see Fig. 5a–c). The optimization of the pyridine capped CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT reference solar cell was made (not shown here) and the optimum ratio for the reference solar cell was found to be 1
P3HT reference solar cell was made (not shown here) and the optimum ratio for the reference solar cell was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. 5d shows the comparison of the cells for each CdSeS nanocrystal with the reference solar cell. Here, the short circuit currents (Isc) and open circuit voltages (Voc) and finally the maximum power values are evaluated. For the L1 capped nanocrystal, a slight improvement in Voc is observed when the P3HT ratio is increased. On the other hand, the corresponding short circuit current of the same solar cell drops by more than half for the direct synthesis method. On the other side, the solar cells fabricated from MC1 and MC2 capped nanocrystals present relatively better results. Especially, the MC1 ligand based device shows a tiny drop in the current when it gives a Voc value of around 500 mV. Unfortunately, the Voc value is rather low for the MC2 ligand based device. Since the MC1 molecule contains two electron accepting cyano groups and a donor carbazole nitrogen, it can easily attract the electrons released by the P3HT material and easily transfer these accepted electrons over to the nanocrystal surface by means of the carbazole unit.
1. Fig. 5d shows the comparison of the cells for each CdSeS nanocrystal with the reference solar cell. Here, the short circuit currents (Isc) and open circuit voltages (Voc) and finally the maximum power values are evaluated. For the L1 capped nanocrystal, a slight improvement in Voc is observed when the P3HT ratio is increased. On the other hand, the corresponding short circuit current of the same solar cell drops by more than half for the direct synthesis method. On the other side, the solar cells fabricated from MC1 and MC2 capped nanocrystals present relatively better results. Especially, the MC1 ligand based device shows a tiny drop in the current when it gives a Voc value of around 500 mV. Unfortunately, the Voc value is rather low for the MC2 ligand based device. Since the MC1 molecule contains two electron accepting cyano groups and a donor carbazole nitrogen, it can easily attract the electrons released by the P3HT material and easily transfer these accepted electrons over to the nanocrystal surface by means of the carbazole unit.
The comparable results are given in Table 1 so that the fabricated devices can be evaluated from their geometries. Results for dark conditions were omitted to prevent confusion in the article. The test measurements clearly indicate that the results of the cells fabricated using the ligand exchange method are better compared to the ones made by direct synthesis. The power conversion efficiencies are summarized in Table 1. We have to notice that the solar cells fabricated in this work consist of spherical nanocrystals which give very low efficiencies as reported by many researchers.18 We just want to present that a new design of the capping molecules may give better results than using the well-known pyridine as the ligand. As we aimed, MC1 capped via the ligand exchange method shows better efficiencies than the reference cell consisting of pyridine capped nanocrystals.
| Device geometry, ITO/PEDOT:PSS/ | QD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT P3HT |
Isc, mA cm−2 | Voc, mV | PCE% |
|---|---|---|---|---|
L1(LE)CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT P3HT |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.27 | 220 | 0.18 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.29 | 280 | 0.26 | |
MC1(LE)CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT P3HT |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.43 | 500 | 0.71 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.47 | 300 | 0.42 | |
MC2(LE)CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT P3HT |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.26 | 380 | 0.30 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.17 | 300 | 0.18 | |
L1(S)CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT P3HT |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.24 | 200 | 0.14 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.25 | 220 | 0.17 | |
MC1(S)CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT P3HT |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.35 | 400 | 0.43 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.3 | 280 | 0.26 | |
MC2(S)CdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT P3HT |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.28 | 220 | 0.19 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.12 | 180 | 0.06 | |
PryCdSeS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P3HT (reference cell) P3HT (reference cell) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.50 | 230 | 0.38 |
We have to emphasize that the role of the new capping ligands in hybrid PV devices should be explained in terms of charge carrier transport or carrier dynamics between the polymer/semiconductor interfaces. But this requires more detailed work based on different techniques such as PIA, ESR, etc. We think that this is subject to further work in future papers.
Conclusions
The fabricated organic solar cell results are better for the ones that are made by the ligand exchange method compared to the ones made by direct synthesis. The device performances for different ratios of L1 ligands are the best for QD and P3HT materials when a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio is used. As for MC1 and MC2 ligand capped nanocrystals, on the other hand, the best results are obtained when 1
3 ratio is used. As for MC1 and MC2 ligand capped nanocrystals, on the other hand, the best results are obtained when 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios of nanocrystal and P3HT are used. Together with L1 attached nanocrystal based devices, a slight development in Voc is observed. On the other hand, the corresponding short circuit current drops by more than half, as shown in Table 1. The MC1 and MC2 molecules give relatively better results. Especially, the MC1 ligand based nanoparticle device shows a slight drop in its current when it gives an open circuit voltage (Voc) of around 500 mV. Consequently, the solar cell performance of new ligands strongly depends on the molecular structure. As we discussed in the previous section, ligand MC1 consisting of both donor and acceptor groups shows the best efficiency.
1 ratios of nanocrystal and P3HT are used. Together with L1 attached nanocrystal based devices, a slight development in Voc is observed. On the other hand, the corresponding short circuit current drops by more than half, as shown in Table 1. The MC1 and MC2 molecules give relatively better results. Especially, the MC1 ligand based nanoparticle device shows a slight drop in its current when it gives an open circuit voltage (Voc) of around 500 mV. Consequently, the solar cell performance of new ligands strongly depends on the molecular structure. As we discussed in the previous section, ligand MC1 consisting of both donor and acceptor groups shows the best efficiency.
Acknowledgements
Dr M. Kus gratefully acknowledges the support from the Scientific and Technical Research Council of Turkey (TUBITAK PN: 113Z254). We also want to thank Selcuk University BAP office for their financial support (PN: 102011151 and 15201098).References
- M. J. Greaney and R. L. Brutchey, Mater. Today, 2015, 18, 31–38 CrossRef CAS.
- W. U. Huynh, J. J. Dittmer and A. P. Alivisatos, Science, 2002, 295, 2425–2427 CrossRef CAS PubMed.
- O. Yagci, S. S. Yesilkaya, S. A. Yüksel, F. Ongül, N. M. Varal, M. Kus, S. Günes and O. Icelli, Synth. Met., 2016, 212, 12–18 CrossRef CAS.
- D. J. Milliron, I. Gur and A. P. Alivisatos, MRS Bull., 2005, 30, 41–44 CrossRef CAS.
- M. D. McGehee, MRS Bull., 2009, 34, 95–100 CrossRef CAS.
- C. Baslak, M. Kus, Y. Cengeloglu and M. Ersoz, J. Lumin., 2014, 153, 177–181 CrossRef CAS.
- M. C. Scharber, D. Muhlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789 CrossRef CAS.
- K. E. Knowles, D. B. Tice, E. A. McArthur, G. C. Solomon and E. A. Weiss, J. Am. Chem. Soc., 2009, 132, 1041–1050 CrossRef PubMed.
- E. Lifshitz, I. Dag, I. Litvitn and G. Hodes, J. Phys. Chem. B, 1998, 102, 9245–9250 CrossRef CAS.
- D. Ginger and N. Greenham, J. Appl. Phys., 2000, 87, 1361–1368 CrossRef CAS.
- M. Kus, F. Ozel, S. Buyukcelebi, A. Aljabour, A. Erdogan, M. Ersoz and N. S. Sariciftci, Opt. Mater., 2015, 39, 103–109 CrossRef CAS.
- N. Radychev, I. Lokteva, F. Witt, J. Kolny-Olesiak, H. Borchert and J. Parisi, J. Phys. Chem. C, 2011, 115, 14111–14122 CAS.
- I. Lokteva, N. Radychev, F. Witt, H. Borchert, J. r. Parisi and J. Kolny-Olesiak, J. Phys. Chem. C, 2010, 114, 12784–12791 CAS.
- J. Y. Lek, L. Xi, B. E. Kardynal, L. H. Wong and Y. M. Lam, ACS Appl. Mater. Interfaces, 2011, 3, 287–292 CAS.
- J. Olson, G. Gray and S. Carter, Sol. Energy Mater. Sol. Cells, 2009, 93, 519–523 CrossRef CAS.
- Y. Zhou, F. S. Riehle, Y. Yuan, H.-F. Schleiermacher, M. Niggemann, G. A. Urban and M. Krüger, Appl. Phys. Lett., 2010, 96, 013304 CrossRef.
- M. Wright and A. Uddin, Sol. Energy Mater. Sol. Cells, 2012, 107, 87–111 CrossRef CAS.
- R. Liu, Materials, 2014, 7, 2747–2771 CrossRef CAS.
- P. Sobrova, M. Ryvolova, J. Hubalek, V. Adam and R. Kizek, Int. J. Mol. Sci., 2013, 14, 13497–13510 CrossRef PubMed.
- E. Kucur, J. Riegler, G. A. Urban and T. Nann, J. Chem. Phys., 2003, 119, 2333–2337 CrossRef CAS.
- E. Aslan, O. Birinci, A. Aljabour, F. Özel, I. Akın, I. Hatay Patir, M. Kus and M. Ersoz, ChemPhysChem, 2014, 15, 2668–2671 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27735c |
| This journal is © The Royal Society of Chemistry 2016 |