Removal of bisphenol A by iron nanoparticle-doped magnetic ordered mesoporous carbon†
Abstract
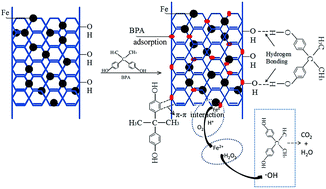
Iron nanoparticle-doped magnetic ordered mesoporous carbon (Fe/OMC) was prepared by co-impregnation and carbothermal reduction methods, and used for highly effective adsorption and degradation of bisphenol A (BPA). Several techniques, including scanning electron microscopy (SEM), transmission electron microscopy (TEM) and nitrogen adsorption–desorption isotherms were applied to characterize the prepared composites. Batch experiments were conducted to explore the decontamination performance, and the results showed that the removal capacity can reach an equilibrium value of 311 mg g−1 at an initial BPA concentration of 200 mg L−1. Kinetic study showed that it agreed well with the pseudo-second-order model (R2 = 0.999). In addition, the Langmuir and Freundlich models were used to describe the removal process. X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) analysis confirmed the existence of Fe0 nanoparticles in the obtained composites. The mechanism of interaction between Fe/OMC and BPA was investigated by Fourier transform infrared spectrometry (FTIR), XRD and XPS analyses. Furthermore, thermodynamics studies were carried out and the exhausted composites could be regenerated with ethanol and easily separated with a magnet.

 Please wait while we load your content...
Please wait while we load your content...