Effect of sulfonated graphene oxide on the performance enhancement of acid–base composite membranes for direct methanol fuel cells
Abstract
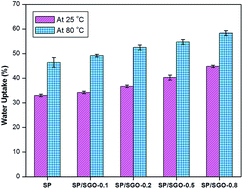
Sulfonated poly(1,4-phenylene ether ether sulfone) (SPEES)/poly(ether imide) (PEI)/sulfonated graphene oxide (SGO) based proton exchange membranes (PEMs) were prepared by a solution casting method. The membranes were characterized for their tensile strength, thermal stability, electrochemical properties and physico-chemical properties using a universal testing machine, thermogravimetric analyzer, impedance spectroscopy and water uptake studies respectively. Compared with SPEES/PEI composite (SP) membranes, the ion exchange capacity, hydrophilicity and water uptake of the SP/SGO membranes were enhanced. Surface morphology of the composite membrane was investigated by atomic force microscopy (AFM) and scanning electron microscopy (SEM). AFM images reveal that the nodule size and surface roughness are increased by the incorporation of SGO. Tensile strength and proton conductivity of the composite membranes increased with increasing SGO content. A maximum conductivity of 8.87 × 10−3 S cm−1 was achieved at 25 °C upon addition of 0.8 wt% of SGO. All the SP/SGO membranes exhibited methanol permeability lower than 3.26 × 10−7 cm2 s−1, which was much lower than that of Nafion 117 (3.41 × 10−6 cm2 s−1). Furthermore, the composite membranes exhibited much higher relative selectivity compared with SP and Nafion 117 membranes. It was found that the SP/SGO-0.8 composite membrane appears to be a good candidate for use in DMFC applications.

 Please wait while we load your content...
Please wait while we load your content...