Chemiluminescence determination of ascorbic acid using graphene oxide@copper-based metal–organic frameworks as a catalyst
Qian Zhu,
Di Dong,
Xuejing Zheng,
Huiqing Song,
Xinrui Zhao,
Hongli Chen and
Xingguo Chen*
State Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metals Chemistry and Resources Utilization of Gansu Province, Department of Chemistry, Lanzhou University, Lanzhou, Gansu 730000, China. E-mail: chenxg@lzu.edu.cn; Fax: +86-931-8912582; Tel: +86-931-8912763
First published on 1st March 2016
Abstract
In this work, composites with different amounts of graphene oxide (GO) and the copper-based metal–organic frameworks (HKUST-1) were synthesized. They have been applied in chemiluminescence (CL) as catalysts using the luminol–H2O2 system as a CL model for the first time. The results showed that the well-dispersed GO@HKUST-1 (GMs) greatly improved the CL intensity of the luminol–H2O2 system, up to 359-fold. Based on ascorbic acid (AA) inhibiting the CL of the luminol–H2O2–GMs system, a flow injection CL method for monitoring AA was developed. Under the optimized conditions, a good linear relationship was obtained from 0.001 μM to 10 μM, and the detection limit was 0.35 nM. Moreover, the method was successfully used to detect AA in commercial food and fruit juices with satisfactory results. Due to their fascinating features, the GMs would offer a suitable catalytic platform for CL systems and provide potential promise for the construction of CL sensors.
Introduction
In recent years, with rising concerns about food safety and health, there have been intense efforts worldwide to find new products with an increased level of health-protecting compounds to meet consumers' needs, especially in search of natural antioxidants.1 L-Ascorbic acid (AA) or vitamin C is the most important water-soluble antioxidant present in food and beverages, and is often used as an antioxidant added to food sources artificially in the food industry. Therefore, it can be applied as a valuable chemical marker for evaluating food deterioration, product quality, and freshness.2 Furthermore, AA is beneficial for cancer prevention and immunity improvement, owing to a significant role in proper functioning of human metabolism and central nervous system.3 However, excess of AA can be metabolized to oxalate, which contributes to a rise in the oxalate level of urine and results in the development of nephrolithiasis in the long run.4,5 Hence, the sensitive determination of AA in foodstuffs and food preservatives is essential for the human health, food assurance, and quality control. So far, numerous analytical methods have been employed to detect and quantify AA, including capillary electrophoresis,6 electrochemistry,7,8 liquid chromatography,9 fluorescence,10 spectrophotometry,11 and chemiluminescence (CL).12,13 Among these methods, CL is a better alternative due to its outstanding features such as excellent sensitivity, wide linear dynamic range, rapid response, simplicity, and low cost.HKUST-1 is one of the most theoretically and experimentally investigated members of the MOFs family.14 Because of its characteristics such as easy synthesis, good hydrophilic, and large porosity, HKUST-1 has become a very prominent catalyst among the various MOFs. Although MOFs show very promising physical and chemical properties for various applications,15–17 their properties can be further improved by specifying the structure of MOFs or chemical modification of surfaces in several ways. Moreover, the catalytic properties can be enhanced via the introduction of other materials.18,19 In a word, MOFs-based composites caused wide attention in the field of analytical chemistry.
Because of its hydrophilic character, graphene oxide (GO) can be easily dispersed in water. Recently, great interest has risen in GO and GO-based composites in the potential application, including electrochemical energy storage,20 catalysis,21,22 as well as sensing.23,24 For example, Huang et al.25 investigated the effects of different carbon nanomaterials as catalysts on the CL of luminol–H2O2 system and the possible CL mechanism. It was found that the GO-enhanced CL is markedly stronger than that of the other carbon materials, because of its higher surface area. Composites based on GO@MOFs enable integration of the unique properties of these two fascinating materials, thus allowing one to design materials which possess the specific properties.26–29 For instance, synergistic effects on porosity and chemistry result in a significant improvement in catalyst.30 In our previous work, we investigated the catalytic CL performance of HKUST-1 for luminol–H2O2 system and the possible CL mechanism.31 It was found that the MOFs can excellently catalyze the CL reaction of luminol–H2O2 system in an alkaline medium. Considering the outstanding catalytic efficiency of both GO and MOFs, we believe that GO@MOFs would provide a promising material for CL investigation. However, to date, most reports have focused primarily on the adsorption capacity,32,33 while few studies have been reported regarding the catalytic CL performance. Considering all of these, the GO@HKUST-1 (GMs) as catalysts for the CL are worth studying, and it was very interesting to investigate the effect of GMs with different GO amounts on CL.
In this work, we prepared the GMs with different GO amounts and firstly investigated their catalytic CL performance for luminol–H2O2 system. The prepared GMs exhibited the outstanding catalytic CL performance. Based on the fact that AA could inhibit the CL signal of luminol–H2O2–GMs system, a sensitive CL detection method for AA has been achieved (Scheme 1). The results showed significant improvements in the sensitivity, detection limit, and range of linear response. Because of the combination of the remarkable features of the GO and HKUST-1, our proposed strategy would open a new avenue to develop the sensitized sensors, especially the CL sensors since GMs exhibited an excellent catalytic CL performance.
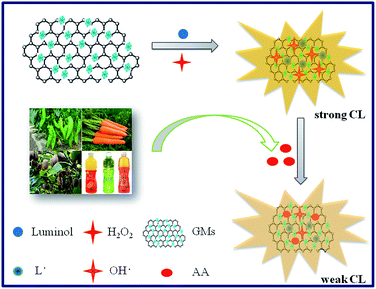 | ||
| Scheme 1 Schematic representation of the sensing strategy of the luminol–H2O2–GMs CL system to detect AA. | ||
Experimental
Chemicals
1,3,5-Benzenetricarboxylic acid, N,N-dimethylformamide (DMF), ethanol and CH2Cl2 were supplied by Rionlon Chemical Reagent Factory (Tianjin, China). Cu(NO3)2·3H2O, H2O2, NaOH, NaHCO3, and Na2CO3 were purchased from Tianjin Guangfu Chemical Reagent Factory (Tianjin, China). Graphite powder and L-ascorbic acid were acquired from Sigma-Aldrich (Sigma-Aldrich, America). A 0.01 M stock solution of luminol (3-aminophthalhydrazide) was prepared by dissolving 0.1772 g of luminol (Sigma-Aldrich, America) in 100 mL of 0.01 M NaOH solution and stored in the dark before use. All reagents were of analytical grade and used without further purification. Deionized water was used throughout the experiments.Apparatus
The CL detection was conducted using an IFFM-E flow-injection CL analyzer (Xi'an, China). A Model TU-1901 double-beam ultraviolet-visible light (UV-vis) spectrophotometer (Beijing Purkine General Instrument Co., Ltd., China) was used to record absorption spectra and measure absorbance. The fluorescent emission spectra were carried out using a Model RF-5301PC spectrofluorophotometer (Shimadzu, Kyoto, Japan). The X-ray diffraction (XRD) measurement was performed using a X'Pertpro Philips X-ray powder diffractometer with Cu Kα radiation (λ = 1.54056 Å). The Fourier transform infrared (FT-IR) spectra were performed using a Nicolet Nexus 670 Fourier transform infrared spectrometer (Nicolet Instrument Co., Madison, WI, USA) using KBr pellets, scanning from 4000 to 500 cm−1 at room temperature. The element determination was measured using Perkin-Elmer Model 4300 DV inductively coupled plasma-atomic emission spectrometer (ICP-AES) equipment (Thermo Jarrel Ash, Franklin, MA, U.S.A.). The electron paramagnetic resonance (EPR) spectra were acquired using a Model JES FA200 spectrometer (JES, Japan). A Model EL20 pH meter (METTLER TOLEDO Instrument Co., Ltd., Shanghai, China) was used to measure pH values.Materials
CL measurements
The CL detection was carried out using a flow-injection CL (FI-CL) system which was fabricated as shown in Fig. 1. The solutions of luminol, H2O2, GMs and buffer/AA were pumped into the flow cell by a peristaltic pump at the rate of 2.6 mL min−1, respectively. The CL signals were monitored by a PMT (−800 V) adjacent to the flow CL cell and then the signal was imported to the computer for data acquisition. The determination of AA was based on the decrease of CL intensity (ΔI = I0 − Is, where I0 and Is were the CL intensity in absence and presence of AA, respectively). | ||
| Fig. 1 Schematic diagram of the FI-CL analysis for the luminol–H2O2–GMs detection system. V: injection valve; W: waste. | ||
Preparation of samples
All the food and fruit juice samples were purchased randomly in the local market in Lanzhou, China. First, the samples were accurately weighed and minced by a mixer. Then, after shaking and ultrasonication for 15 min, the mixture was filtered with a syringe membrane filter. The samples were directly diluted to an appropriate concentration with deionized water before FI-CL analysis. All the samples were stored in a refrigerator at 4 °C for use.Results and discussion
Characterization
Five different GMs were prepared by a solvothermal method with different amounts of GO. To verify the successful synthesis, various characterization methods, including XRD, FT-IR, Raman spectroscopy, and ICP-AES analysis, were employed. Fig. 2A provides the X-ray diffraction patterns of the parent materials as well as the composites, GM-1, GM-2, GM-3, GM-4, and GM-5. For HKUST-1 (GM-0), the characteristic diffraction peaks at 2θ of ∼9.52°, 11.68°, 13.46°, 17.50°, and 19.08° were assigned to the (110), (222), (400), (333), and (440) planes, which were characteristic of this material structure and was in accordance with the data reported in the literature.31,35 Other interesting features, there were several splitting peaks for GM-5 due to the high GO amount of 37%, and these splitting peaks increased with an increase in the amounts of GO in GMs.29,36 However, it should be noted that the diffraction patterns of the GMs did not differ significantly from that for GM-0 because HKUST-1 was their major composition, which suggested that the GMs preserved the major structural and chemical features of HKUST-1. In other words, the GO did not prevent or destroy the formation of HKUST-1 in the composites, as mentioned in some other pioneering work.32,37 The FT-IR spectra were used to confirm the structure and composition of GMs (Fig. 2B). The spectrum of GM-0 matched well with that reported in the literature,38 and the spectra of GMs exhibited the features similar to that of GM-0. The reason was that HKUST-1 represented the major component of the composites. It also suggested that the GMs preserved the crystalline characters of parental HKUST-1. More precisely, the bands originated from the asymmetric (1450 and 1370 cm−1) and symmetric (1645 and 1590 cm−1) stretching vibrations of the carboxylate groups in BTC (1,3,5-benzenetricarboxylate), respectively. Below 1300 cm−1, various bands were assigned to the out-of-plane vibrations of BTC.32,39 In addition, the band at 1700 cm−1 was associated with stretching of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of carbonyl or carboxyl groups. Deformation of the C–O bond was observed as the band at 1070 cm−1.40 The results illustrated the presence of GO. Overall, all the characteristics peaks above were also observed in GMs, confirming that the structure of HKUST-1 was preserved. Raman spectroscopy is a widely used tool for the characterization of GO. As can be seen from Fig. 2C, two typical features bands were observed on the spectra of the GMs: the G band at 1593 cm−1 and the D band at 1355 cm−1, demonstrating the existence of the GO structure in the GMs.41,42 The intensity ratio of the G and D bands (IG/ID) provides an indication of the disorder in the material studied.43 Furthermore, it is interesting to see that the marked change in the appearance of the GMs with different amounts of GO incorporated in the HKUST-1 structure. The GMs with the highest GO amounts appeared much darker than the ones with low GO amounts (Fig. 2D). Finally, to further verify the detailed composition of GMs, the amounts of GO in GMs can be indirectly found out by means of ICP analysis. The results of ICP-AES analysis showed that the amount of Cu in the GM-0 was 27.52 wt%. The decline of the amounts of Cu in the GMs mainly resulted from the introduction of the GO in comparison with the GM-0. Therefore, according to the reported literature,37 the amounts of GO in GMs were calculated and varied from 1.0 to 37.0 wt% (Table 1). Taken together, from the above observations, GMs were successfully prepared.
O bond of carbonyl or carboxyl groups. Deformation of the C–O bond was observed as the band at 1070 cm−1.40 The results illustrated the presence of GO. Overall, all the characteristics peaks above were also observed in GMs, confirming that the structure of HKUST-1 was preserved. Raman spectroscopy is a widely used tool for the characterization of GO. As can be seen from Fig. 2C, two typical features bands were observed on the spectra of the GMs: the G band at 1593 cm−1 and the D band at 1355 cm−1, demonstrating the existence of the GO structure in the GMs.41,42 The intensity ratio of the G and D bands (IG/ID) provides an indication of the disorder in the material studied.43 Furthermore, it is interesting to see that the marked change in the appearance of the GMs with different amounts of GO incorporated in the HKUST-1 structure. The GMs with the highest GO amounts appeared much darker than the ones with low GO amounts (Fig. 2D). Finally, to further verify the detailed composition of GMs, the amounts of GO in GMs can be indirectly found out by means of ICP analysis. The results of ICP-AES analysis showed that the amount of Cu in the GM-0 was 27.52 wt%. The decline of the amounts of Cu in the GMs mainly resulted from the introduction of the GO in comparison with the GM-0. Therefore, according to the reported literature,37 the amounts of GO in GMs were calculated and varied from 1.0 to 37.0 wt% (Table 1). Taken together, from the above observations, GMs were successfully prepared.
 | ||
| Fig. 2 Characterization of catalysts. (A) X-ray diffraction patterns. (B) FT-IR spectra. (C) Raman spectra. (D) Pictures ((a) GM-0; (b) GM-1; (c) GM-2; (d) GM-3; (e) GM-4; and (f) GM-5). | ||
| Sample | GO amounts (wt%) | Element (wt%) |
|---|---|---|
| GM-0 (HKUST-1) | 0 | Cu 27.52; C 29.56; H 2.34 |
| GM-1 | 1.00 | Cu 27.39; C 26.64; H 1.94 |
| GM-2 | 5.00 | Cu 26.14; C 28.06; H 2.02 |
| GM-3 | 6.00 | Cu 25.94; C 30.29; H 1.93 |
| GM-4 | 8.00 | Cu 25.40; C 28.73; H 1.86 |
| GM-5 | 37.00 | Cu 17.35; C 35.98; H 2.51 |
CL performance of the GMs
The CL kinetic curves after addition of GMs to luminol–H2O2 system were investigated by the FI-CL analysis (Fig. 3). As shown Fig. 3A, GMs greatly improved the CL intensity of luminol–H2O2 system, and among the GMs studied in the present work, the maximum CL intensity obtained by GM-2 as catalyst was up to 359-fold enhancement compared with the luminol–H2O2 system under the same experimental conditions, which was also higher than that of other GMs. Thereby in our present work, GM-2 was chosen as the catalyst to improve the sensitivity of the prepared sensor. Furthermore, from Fig. 3B, the luminol oxidation by H2O2 generated a weak CL signal in alkaline media (curve a). In contrast, very bright CL emission was observed as soon as GM-2 was added into luminol–H2O2 solution (curve b). Therefore, the enhanced CL intensity could be attributed to the addition of GM-2 into the mixture solution of luminol–H2O2. To investigate the effect of antioxidant on the CL intensity of luminol–H2O2–GM-2 system, AA as a representative analyte was added into the solution (curve c). Evidently, addition of AA resulted in a drastic reduction of CL intensity. Moreover, the kinetic curve of luminol–H2O2–GM-2 was similar to the luminol–H2O2 reaction, which showed that the CL intensity reached a maximum value under the catalysis of GM-2.Optimization of the experimental conditions
In order to obtain the best sensor performance, the effects of chemical parameters, including pH of buffer solution (Na2CO3–NaHCO3), catalyst dosage, and the concentrations of luminol and H2O2, on the CL intensity of the luminol–H2O2–GM-2 system were studied.It is well known that the luminol–H2O2 CL reaction often occurres in basic conditions, and the pH is an important factor influencing the CL reaction, thus the pH in the range of 10.0–11.5 was evaluated. As depicted in Fig. 4A, the CL intensity increased with increasing the pH of buffer solution and eventually reached its maximum at pH 10.5, which was attributed to the faster generation of effective hydroxyl radical (OH˙) from H2O2 at higher pH,44 thus increasing the CL intensity. However, the CL intensity decreased significantly when the pH was above 10.5, possibly due to the fact that the catalytic ability of GM-2 declined in the high alkaline media.45,46 Finally, pH 10.5 was chosen for the further work.
Next, the effect of the catalyst dosage in the range of 5–60 μg mL−1 was studied (Fig. 4B). The CL intensity increased with increasing the catalyst dosage, until it eventually reached a plateau at 50 μg mL−1. Therefore, in the following experiments, 50 μg mL−1 was selected as the optimum catalyst dosage.
Finally, the effect of the concentration of luminol and H2O2 were examined. The results were shown in Fig. 4C and D. For luminol, the concentration in the range of 0–8 μM was optimized, and the results demonstrated that with increasing the concentration of luminol, the CL intensity also increased. The strong CL intensity was attributed to the fact that the number of luminol radical (L˙) increased with increasing concentration, which was beneficial to enhance the CL intensity and thereby improving the sensitivity of the CL system. However, the trend of slowing growth was appeared when the concentration of luminol was higher than 2.5 μM. Considering the CL intensity and the consumption of the reagents, 2.5 μM was the optimized concentration of luminol. For H2O2, the concentration in the range of 0–15 μM was investigated and the results showed that the CL intensity increased steadily with increasing the concentration of H2O2, which resulted from the rising number of OH˙. Taking account of the instability of H2O2, the concentration of H2O2 was 10 μM for the following research work.
In a word, the optimized conditions for the luminol–H2O2–GM-2 CL system were as follows: the pH of buffer was 10.5, catalyst dosage and the concentrations of luminol and H2O2 were 50 μg mL−1, 2.5 μM and 10 μM, respectively.
Possible mechanism
For investigating the high catalytic performance of GM-2, the effect of GO, HKUST-1, and GM-2 on CL intensity were studied, respectively. As shown in Fig. 5, the CL intensity of luminol–H2O2 system in the presence of GM-2 was about 359 times stronger than that of luminol–H2O2 system and were also much higher than that of both GO and HKUST-1, respectively. Moreover, the CL intensity when GM-2 presented was far more than the sum of the both intensity of GO and HKUST-1, indicating that the synergistic effect between GO and HKUST-1 was existed, which accounted for the observed high catalytic activity. | ||
| Fig. 5 CL spectra of (a) luminol–H2O2, (b) luminol–H2O2–GO, (c) luminol–H2O2–HKUST-1, and (d) luminol–H2O2–GM-2. | ||
To better clarify the catalytic performance of GM-2, the UV-vis absorption spectrum change of GM-2 was investigated. As illustrated in Fig. 6, two absorption bands at 297 and 348 nm were observed in the luminol–H2O2 system, respectively, corresponding to the absorption peak of luminol, while GM-2 only has a maximum absorption band at 280 nm. They clearly indicated that the maximum absorption wavelength between GM-2 and luminol–H2O2–GM-2 system showed no significant difference, indicating that there were no new species generated after injection of GM-2. Therefore, it is proved that GM-2 can act as a catalyst in luminol–H2O2–GM-2 system.
 | ||
| Fig. 6 UV-visible absorption spectra of (a) luminol, (b) luminol–H2O2, (c) GM-2, and (d) luminol–H2O2–GM-2. | ||
In order to identify the emission species during the CL reaction, the CL spectrum of the luminol–H2O2–GM-2 system was measured through a spectrofluorimeter without excitation light source (Fig. 7). It could be clearly seen from Fig. 7 that there was only one emission band in the range of 325–550 nm with a maximum emission peak at ∼425 nm, meaning that the emitter of the present system was still 3-aminophthalate, which was consistent with the CL spectrum of the luminol–H2O2 system (curve a).25 In addition, very bright CL emission was observed when GM-2 was added into luminol–H2O2 solution (curve b), showing that GM-2 can strongly catalyze the CL reaction of the luminol–H2O2 system. In a word, the role of GM-2 only acted as an enhancement catalytic reagent, which was consistent with those described nanocatalysis on the luminol–H2O2 system previously.31,47–49
Furthermore, the formation of intermediates could be identified by room-temperature EPR spectroscopy. The H2O2–GM-2 system was employed to confirm the existence of OH˙. Fig. 8 presents the signal change of 5,5-dimethyl-1-pyrroline N-oxide (DMPO)/OH˙ adduct with the different concentration of GM-2, revealing that the OH˙ formed in H2O2 solution through the addition of GM-2 and that the amount of free radical was proportional to the dosage of GM-2. In addition, as an assistant validation method, quenching CL experiments with the addition of the radical scavengers were obtained. AA is well-known as an effective radical scavenger for OH˙.50 As shown in Fig. 9, the CL intensity quickly reduced with the increasing concentrations of AA, demonstrating that OH˙ was indeed involved in the CL reaction. This result was in agreement with the obtained result by EPR. These results suggested that GM-2 possessed the catalytic ability to decompose H2O2 to generate OH˙, and the resulting OH˙ then reacted with luminol to facilitate the generation of L˙, which further reacted with monodissociated H2O2, giving rise to light emission.
 | ||
| Fig. 8 DMPO spin-trapping EPR spectra of OH˙ in the H2O2–GM-2 system, (a) 0 μg mL−1, (b) 4 μg mL−1, and (c) 8 μg mL−1 of GM-2, respectively. | ||
 | ||
| Fig. 9 Response and calibration curves (inset) of AA standard solutions under the optimized conditions. | ||
Taken together, the catalytic mechanism of GM-2 for luminol–H2O2 system was attributed to two aspects: the synergistic effect between GO and HKUST-1 through the electron transfer30 and facilitating the decomposition of H2O2 to generate OH˙ owing to the GM-2 porous structure.31
Analytical performance
As indicated in Fig. 9, the decrease of CL intensity was closely relevant to the concentration of AA under the optimum conditions described above. The inset image shows the linear plot of the logarithm of ΔI versus the logarithm of AA concentration over the range of 0.001–10 μM. The regression equation was log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ΔI = 0.2399
ΔI = 0.2399![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C + 3.9811 (R2 = 0.9935), where ΔI was the variation between I0 and Is, and C was the concentration of AA. The limit of detection for AA was 0.35 nM at a signal-to-noise ratio of 3. The relative standard deviation (RSD) was 2.2% for twelve repeated measurements of 0.1 μM AA, indicating an acceptable reproducibility for AA detection. Finally, in order to make a comprehensive evaluation on the advantages of the presently developed sensor, the present results were compared to those in literature (Table 2).12,13,51–53 It can be known from the comparison that the linear response range (5 order of magnitude) of present AA sensor is excellent among AA sensors, and the LOD (0.35 nM) of the presently developed AA sensor can be comparable or even better than most of the previous reported AA sensors. It should be noted that the linear range was the same and the LOD was lower in system of Luminol–K3Fe(CN)6–GNPs.53 However, in this work, it is for the first time to report the catalytic CL performance of GMs and propose the sensitive sensing platform for AA detection based on the luminol–H2O2–GMs system. The present study not only provides a feasible way to enrich the traditional luminol CL catalyst, but also broadens the application of catalyst-functionalized MOFs for CL sensing.
C + 3.9811 (R2 = 0.9935), where ΔI was the variation between I0 and Is, and C was the concentration of AA. The limit of detection for AA was 0.35 nM at a signal-to-noise ratio of 3. The relative standard deviation (RSD) was 2.2% for twelve repeated measurements of 0.1 μM AA, indicating an acceptable reproducibility for AA detection. Finally, in order to make a comprehensive evaluation on the advantages of the presently developed sensor, the present results were compared to those in literature (Table 2).12,13,51–53 It can be known from the comparison that the linear response range (5 order of magnitude) of present AA sensor is excellent among AA sensors, and the LOD (0.35 nM) of the presently developed AA sensor can be comparable or even better than most of the previous reported AA sensors. It should be noted that the linear range was the same and the LOD was lower in system of Luminol–K3Fe(CN)6–GNPs.53 However, in this work, it is for the first time to report the catalytic CL performance of GMs and propose the sensitive sensing platform for AA detection based on the luminol–H2O2–GMs system. The present study not only provides a feasible way to enrich the traditional luminol CL catalyst, but also broadens the application of catalyst-functionalized MOFs for CL sensing.
| System | Linear range (μM) | LOD (nM) | Sample | Ref. |
|---|---|---|---|---|
| Luminol–H2O2–gold colloids | 0.005–5.0 | 2.0 | Not given | 51 |
| Luminol–H2O2–cation exchange resin | 0.02–0.6 | 6.0 | Urine | 52 |
| Luminol–K3Fe(CN)6–GNPs | 0.0001–1.0 | 0.02 | Fruit juice and vitamin C tablets | 53 |
| Mg–Al–CO3 LDHs-catalyzed ONOOH | 0.005–5.0 | 0.5 | Commercial liquid fruit juices | 12 |
| NaHCO3–H2O2–CdSe/CdS QDs | 0.1–100 | 6.7 | Human serum | 13 |
| Luminol–H2O2–GMs | 0.001–10 | 0.35 | Food and fruit juice | This work |
Interference studies
Generally, the interference from co-existing chemical components present in real samples and structuralanalogue should be investigated in the detection of AA. Therefore, the effects of co-existing chemical components were monitored and compared to that of AA (0.1 μM). When errors produced by co-existing chemical components were within ±5%, the ratios of co-existing chemical components tolerated in analysis of AA were 100-fold for NO2−, Mg2+, K+, Ca2+, Na+, Cl−, NH4+, NO3−, Zn2+, SO42−, 50-fold for Cu2+, glucose, 10-fold for SO32−, and 5-fold for fructose. Moreover, it is worth mentioning that uric acid (UA) and dopamine (DA) interfere in the determination of AA,8,54 but the aim of this work is to determine AA in real samples of food and fruit juices by the present system, the interference originated from UA and DA did not study. In addition, the high selectivity was also attributed to the fact that AA is well-known as an effective radical scavenger for OH˙, which is the important radical during the light emission in this work. The results summarized in Fig. 10 and demonstrated that the proposed CL sensor based on the luminol–H2O2–GM-2 system was appropriate for the practical application of AA with minor interference.Analytical application
Foodstuffs are a good exogenous source of natural antioxidants and the antioxidant capacity of food samples is usually used to evaluate their health-beneficial effects. Therefore, in this study AA as a representative antioxidant was detected because of abundant amounts in food and fruit juices. The results of AA detection in six types of food and fruit juices were shown in Table 3. Furthermore, the recovery tests were obtained by adding a series of different known concentration of AA. As the results summarized in Table 3, the average recoveries of AA in green pepper, carrot, kiwi fruit, orange juice, crystal grape, and juicy peach were found to be 103.0%, 88.0%, 91.0%, 97.0%, 99.0%, and 97.0%, respectively. These results confirmed that the proposed method had a great potential application in the antioxidant determination of commercial food and fruit juices.| Sample | Initial amount (g 100 g−1) | Added amount (μM) | Recovery (%) | Average recovery (%) | RSD (n = 3, %) |
|---|---|---|---|---|---|
| Green pepper | 0.062 | 0.050 | 110.0 | 103.0 | 1.6 |
| 0.50 | 108.0 | 0.9 | |||
| 1.0 | 91.0 | 0.9 | |||
| Carrot | 0.015 | 0.010 | 93.0 | 88.0 | 0.3 |
| 0.10 | 82.0 | 0.6 | |||
| 0.50 | 88.0 | 3.3 | |||
| Kiwi fruit | 0.062 | 0.050 | 85.0 | 91.0 | 1.9 |
| 0.10 | 94.0 | 1.9 | |||
| 0.50 | 95.0 | 2.0 | |||
| Orange juice | 0.13 | 0.050 | 84.0 | 97.0 | 1.4 |
| 0.50 | 93.0 | 2.9 | |||
| 1.0 | 113.0 | 1.0 | |||
| Crystal grape | 0.20 | 0.10 | 89.0 | 99.0 | 2.1 |
| 0.50 | 107.0 | 0.1 | |||
| 1.0 | 100.0 | 2.1 | |||
| Juicy peach | 0.14 | 0.050 | 106.0 | 97.0 | 0.9 |
| 0.10 | 97.0 | 2.1 | |||
| 0.50 | 88.0 | 2.9 |
Conclusions
In summary, GMs catalysts have been synthesized and were first demonstrated to exhibit superior catalytic properties for enhancing the CL intensity of luminol–H2O2 system. The effects of buffer pH, catalyst dosage, and concentrations of luminol and H2O2 on the CL intensity of the luminol–H2O2–GMs system have been systematically investigated. In addition, the catalytic mechanism was also studied. Furthermore, the synthesized GMs catalysts have been used to develop practical sensors for AA content in food and fruit juices samples with satisfactory results, indicating that catalyst-functionalized MOFs for CL sensing would have promising applications in the future.Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China (No. 21375053) and Special Doctorial Program Fund from the Ministry of Education of China (No. 20130211110039).Notes and references
- A. M. Martín-Sánchez, S. Cherif, J. Ben-Abda, X. Barber-Vallés, J. Á. Pérez-Álvarez and E. Sayas-Barberá, Food Chem., 2014, 158, 513–520 CrossRef PubMed.
- Z. Gazdik, O. Zitka, J. Petrlova, V. Adam, J. Zehnalek, A. Horna, V. Reznicek, M. Beklova and R. Kizek, Sensors, 2008, 8, 7097–7112 CrossRef CAS.
- Q. W. Lian, Z. F. He, Q. He, A. Luo, K. W. Yan, D. X. Zhang, X. Q. Lu and X. B. Zhou, Anal. Chim. Acta, 2014, 823, 32–39 CrossRef CAS PubMed.
- S. Y. Qiu, S. Gao, L. D. Xie, H. Q. Chen, Q. D. Liu, Z. Y. Lin, B. Qiu and G. N. Chen, Analyst, 2011, 136, 3962–3966 RSC.
- A. C. Baxmann, C. D. Mendonça and I. P. Heilberg, Kidney Int., 2003, 63, 1066–1071 CrossRef CAS PubMed.
- K. Fukushi, S. Takeda, S. Wakida, M. Yamane, K. Higashi and K. Hiiro, J. Chromatogr., 1997, 772, 313–320 CrossRef CAS.
- J. F. Cabrita, V. C. Ferreira and O. C. Monteiro, Electrochim. Acta, 2014, 135, 121–127 CrossRef CAS.
- N. Moghimi and K. T. Leung, Anal. Chem., 2013, 85, 5974–5980 CrossRef CAS PubMed.
- L. Nováková, P. Solich and D. Solichová, TrAC, Trends Anal. Chem., 2008, 27, 942–958 CrossRef.
- X. Wu, Y. X. Diao, C. X. Sun, J. H. Yang, Y. B. Wang and S. N. Sun, Talanta, 2003, 59, 95–99 CrossRef CAS PubMed.
- L. P. Zhang, B. Hu and J. H. Wang, Anal. Chim. Acta, 2012, 717, 127–133 CrossRef CAS PubMed.
- Z. H. Wang, X. Teng and C. Lu, Analyst, 2012, 137, 1876–1881 RSC.
- H. Chen, R. B. Li, L. Lin, G. S. Guo and J. M. Lin, Talanta, 2010, 81, 1688–1696 CrossRef CAS PubMed.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed.
- S. C. Yang, F. G. Ye, Q. H. Lv, C. Zhang, S. F. Shen and S. L. Zhao, J. Chromatogr., 2014, 1360, 143–149 CrossRef CAS PubMed.
- T. R. C. Van Assche, T. Duerinck, S. Van der Perre, G. V. Baron and J. F. M. Denayer, Langmuir, 2014, 30, 7878–7883 CrossRef CAS PubMed.
- D. Y. Lee, D. V. Shinde, S. J. Yoon, K. N. Cho, W. Lee, N. K. Shrestha and S. Han, J. Phys. Chem. C, 2014, 118, 16328–16334 CAS.
- Y. F. Chen, X. Q. Huang, X. Feng, J. K. Li, Y. Y. Huang, J. S. Zhao, Y. X. Guo, X. M. Dong, R. D. Han, P. F. Qi, Y. Z. Han, H. W. Li, C. W. Hu and B. Wang, Chem. Commun., 2014, 50, 8374–8377 RSC.
- Y. F. Zhang, X. J. Bo, C. Luhana, H. Wang, M. Li and L. P. Guo, Chem. Commun., 2013, 49, 6885–6887 RSC.
- H. Gwon, H. Kim, K. U. Lee, D. Seo, Y. C. Park, Y. Lee, B. T. Ahn and K. Kang, Energy Environ. Sci., 2011, 4, 1277–1283 CAS.
- Y. L. Dong, H. G. Zhang, Z. U. Rahman, L. Su, X. J. Chen, J. Hu and X. G. Chen, Nanoscale, 2012, 4, 3969–3976 RSC.
- N. N. Zhang, H. X. Qiu, Y. Liu, W. Wang, Y. Li, X. D. Wang and J. P. Gao, J. Mater. Chem., 2011, 21, 11080–11083 RSC.
- J. Bai and X. E. Jiang, Anal. Chem., 2013, 85, 8095–8101 CrossRef CAS PubMed.
- Q. W. Chen, L. Y. Zhang and G. Chen, Anal. Chem., 2012, 84, 171–178 CrossRef CAS PubMed.
- D. M. Wang, Y. Zhang, L. L. Zheng, X. X. Yang, Y. Wang and C. Z. Huang, J. Phys. Chem. C, 2012, 116, 21622–21628 CAS.
- Y. X. Zhao, M. Seredych, Q. Zhong and T. J. Bandosz, ACS Appl. Mater. Interfaces, 2013, 5, 4951–4959 CAS.
- S. Liu, L. X. Sun, F. Xu, J. Zhang, C. L. Jiao, F. Li, Z. B. Li, S. Wang, Z. Q. Wang, X. Jiang, H. Y. Zhou, L. N. Yang and C. Schick, Energy Environ. Sci., 2013, 6, 818–823 CAS.
- C. Petit, L. L. Huang, J. Jagiello, J. Kenvin, K. E. Gubbins and T. J. Bandosz, Langmuir, 2011, 27, 13043–13051 CrossRef CAS PubMed.
- C. Petit and T. J. Bandosz, Adv. Mater., 2009, 21, 4753–4757 CrossRef CAS.
- M. Jahan, Z. L. Liu and K. P. Loh, Adv. Funct. Mater., 2013, 23, 5363–5372 CrossRef CAS.
- Q. Zhu, Y. L. Chen, W. F. Wang, H. G. Zhang, C. L. Ren, H. L. Chen and X. G. Chen, Sens. Actuators, B, 2015, 210, 500–507 CrossRef CAS.
- C. Petit, B. Mendoza, D. O'Donnell and T. J. Bandosz, Langmuir, 2011, 27, 10234–10242 CrossRef CAS PubMed.
- C. Petit, J. Burress and T. J. Bandosz, Carbon, 2011, 49, 563–572 CrossRef CAS.
- M. Seredych and T. J. Bandosz, J. Phys. Chem. C, 2007, 111, 15596–15604 CAS.
- X. W. Zhang, W. J. Dong, Y. Luan, M. Yang, L. Tan, Y. G. Guo, H. Y. Gao, Y. H. Tang, R. Dang, J. Li and G. Wang, J. Mater. Chem. A, 2015, 3, 4266–4273 CAS.
- C. Petit and T. J. Bandosz, J. Mater. Chem., 2009, 19, 6521–6528 RSC.
- X. Zhou, W. Y. Huang, J. Shi, Z. X. Zhao, Q. B. Xia, Y. W. Li, H. H. Wang and Z. Li, J. Mater. Chem. A, 2014, 2, 4722–4730 CAS.
- Y. Seo, G. Hundal, I. T. Jang, Y. K. Hwang, C. Jun and J. Chang, Microporous Mesoporous Mater., 2009, 119, 331–337 CrossRef CAS.
- D. D. Zu, L. Lu, X. Q. Liu, D. Y. Zhang and L. B. Sun, J. Phys. Chem. C, 2014, 118, 19910–19917 CAS.
- M. Herrera-Alonso, A. A. Abdala, M. J. McAllister, I. A. Aksayand and R. K. Prud’homme, Langmuir, 2007, 23, 10644–10649 CrossRef CAS PubMed.
- K. N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prud’homme, I. A. Aksay and R. Car, Nano Lett., 2008, 8, 36–41 CrossRef CAS PubMed.
- Y. F. Yang, J. Wang, J. Zhang, J. C. Liu, X. L. Yang and H. Y. Zhao, Langmuir, 2009, 25, 11808–11814 CrossRef CAS PubMed.
- F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 1126–1130 CrossRef CAS.
- Y. Nosaka, Y. Yamashita and H. Fukuyama, J. Phys. Chem. B, 1997, 101, 5822–5827 CrossRef CAS.
- D. L. Giokas, A. G. Vlessidis, G. Z. Tsogas and N. P. Evmiridis, TrAC, Trends Anal. Chem., 2010, 29, 1113–1126 CrossRef CAS.
- B. Haghighi and S. Bozorgzadeh, Microchem. J., 2010, 95, 192–197 CrossRef CAS.
- W. Chen, L. Hong, A. L. Liu, J. Q. Liu, X. H. Lin and X. H. Xia, Talanta, 2012, 99, 643–648 CrossRef CAS PubMed.
- S. H. He, W. B. Shi, X. D. Zhang, J. Li and Y. M. Huang, Talanta, 2010, 82, 377–383 CrossRef CAS PubMed.
- L. J. Zhang, Y. C. Chen, Z. M. Zhang and C. Lu, Sens. Actuators, B, 2014, 193, 752–758 CrossRef CAS.
- Y. He, X. He, X. Y. Liu, L. F. Gao and H. Cui, Anal. Chem., 2014, 86, 12166–12171 CrossRef CAS PubMed.
- Z. F. Zhang, H. Cui, C. Z. Lai and L. J. Liu, Anal. Chem., 2005, 77, 3324–3329 CrossRef CAS PubMed.
- L. F. Cai and C. X. Xu, J. Chil. Chem. Soc., 2011, 56, 938–940 CrossRef CAS.
- Y. P. Dong, T. T. Gao, X. F. Chu, J. Chen and C. M. Wang, J. Lumin., 2014, 154, 350–355 CrossRef CAS.
- G. H. Wu, Y. H. Wu, X. W. Liu, M. C. Rong, X. M. Chen and X. Chen, Anal. Chim. Acta, 2012, 745, 33–37 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |




