Two-dimensional graphene-like MoSe2 nanosheets anchored on hollow carbon nanofibers as a cathode catalyst for rechargeable Li–O2 batteries
Yanqing Lai,
Wei Chen,
Zhian Zhang*,
Yongqing Gan,
Xing Yang and
Jie Li
School of Metallurgy and Environment, Central South University, Changsha, Hunan 410083, China. E-mail: zhangzhian@csu.edu.cn; Tel: +86 731 88830649
First published on 9th February 2016
Abstract
MoSe2@HCNF hybrids are synthesized via a facile hydrothermal method. The as-synthesized MoSe2@HCNF hybrids are used as the cathode catalyst of Li–O2 batteries for the first time. The successful growth of MoSe2 nanosheets on the surface of HCNF mitigates the agglomeration of MoSe2 nanosheets and results in more edge sites exposed. And the electronic conductivity of the hybrids is enhanced by the combination of HCNF. Therefore, the MoSe2@HCNF hybrids exhibit superior catalytic activity, especially the OER catalytic activity for the charge process. The charge over-potential of the Li–O2 batteries is greatly reduced, delivering a charge voltage platform of ∼3.69 V at 0.1 mA cm−2, and the cycle performance is improved. According to this study, it can be expected that metal selenides have a great potential to be used as the cathode catalyst for Li–O2 batteries.
Introduction
With the development of portable electronic devices and the exploitation of the electric vehicle market, there is a growing demand for the energy density of secondary batteries. For their ultrahigh specific energy density, Li–O2 batteries are found to be the most promising new kind of rechargeable battery.1–3 However, there are still many problems preventing the practical applications of Li–O2 batteries, such as poor rate performance and stability, especially the high over-potential during the OER process of the cathode that relates to the generation and decomposition of Li2O2.4–6 The addition of catalyst into the cathode is considered to be the most efficient method to overcome these challenges.7–9In order to explore alternatives to the precious metal catalysts, which are scarce and expensive, many kinds of low-cost catalysts are researched, including metal oxide,7,9 nitride,8 carbides10 and sulfides.11 As their performances are not satisfactory, it is still necessary to develop new types of catalyst materials. Recently, lots of studies have demonstrated that selenium compounds showed excellent catalytic activity in various applications, such as water splitting,12,13 hydrogen generation14 and fuel cell.15 Among them, MoSe2 is attractive in terms of its two-dimensional (2D) graphene-like structure,13,16,17 which possesses high electronic conductivity in the monolayer of the (002) plane. And the exposed edge sites of the (002) plane of MoSe2 exhibit particularly good catalytic ability.13,15,18 However, there have no reports about MoSe2 used as the catalyst of Li–O2 batteries. If some appropriate improvements can be implemented, the performance of MoSe2 in Li–O2 batteries will be worth looking forward to.
Here, in our work, MoSe2 are grown on the surface of hollow carbon nanofibers (HCNF). By using the orientation growth of MoSe2 on the carbon materials, the MoSe2@HCNF hybrids, which have more edge sites of the (002) plane exposed, was obtained. In addition, the high electronic conductivity of HCNF can improve the electrical contact between different MoSe2 nanosheets and is beneficial to the transmission of electron from active sites to electrode. While used as the catalyst of Li–O2 batteries, MoSe2@HCNF exhibits excellent electrochemical properties.
Experiment
Synthesis of MoSe2@HCNF composite
MoSe2@HCNF was synthesized by the similar method described in our previous work.19 4 mmol of Se powders was dissolved into 10 ml hydrazine hydrate by sonication. After standing for 24 h, the reaction solution A was obtained. And 2 mmol of NaMoO4 was dissolved in the mixed solution of deionized water and ethanol by sonication. After 0.08 g of HCNF was uniformly dispersed into this mixed solution by sonication, the reaction solution B was prepared. These two reaction solutions were mixed in a Teflon autoclave. After the autoclave was placed in an oven at 200 °C for 10 h, it was cooled down to room temperature. Then, the black precipitate in the Teflon lining was collected by vacuum filtration and purified by hot water and ethanol for 3 times, respectively. After drying in a vacuum oven at 70 °C for 24 h, the black precipitate was annealed at 600 °C for 2 h under Ar atmosphere and the final product MoSe2@HCNF was obtained. For comparison, the pure MoSe2 was prepared via the same process described above without the addition of HCNF into the reaction solvent B.Materials characterizations
The powder X-ray diffraction (XRD) patterns were obtained by using a Rigaku3014 equipped with a graphite-monochromated Cu Kα radiation source. Raman spectra (Raman) was measured on a Jobin-Yvon LabRAM HR-800 Raman spectrometer. Field emission scanning electron microscopy (SEM) images were obtained by using Nova NanoSEM 230. Transmission electron microscopy (TEM) images and the energy-dispersive X-ray spectroscopy (EDX) were taken on Tecnai G2 20ST. N2 adsorption/desorption measurements were performed by using a Quantachrome instrument (Quabrasorb SI-3MP) at 77 K. X-ray photoelectron spectroscopy (XPS) was obtained by using a ThermoFisher ESCALAB250xi.Electrochemical tests
Electrochemical characterizations were carried out in Li–O2 coin batteries, drilled with 3 × Φ1.5 mm holes in the center of the cell pans in an evenly distributed pattern to allow O2 passage, consisting of a Li foil anode, an electrolyte of 1 M LiTFSI in tetraethylene glycol dimethyl ether (TEGDME), and an O2 electrode (10 mm in diameter). The O2 electrodes were prepared by casting a mixture containing as-prepared MoSe2@HCNF (36 wt%), Super P (54 wt%) and PVDF (10 wt%) onto a Ni foam current collector, followed by drying at 50 °C in a vacuum oven for 12 h. The same method was employed to prepare the electrodes with pure MoSe2 which consist of MoSe2 (36 wt%), Super P (54 wt%) and PVDF (10 wt%) and the pure Super P electrodes which consist of Super P (90 wt%) and PVDF (10 wt%). The Li–O2 cells were assembled in an Ar filled glove box (Universal 2440/750) in which oxygen and water contents were less than 1 ppm. The galvanostatic discharge/charge performance of the Li–O2 battery was tested at a current density of 0.1–0.5 mA cm−2 in the potential range from 2.0 to 4.4 V under a LAND CT2001A system. It is noted that the specific capacity was calculated based on the total mass of carbon and catalyst. All measurements were conducted in 1 atm dry oxygen to avoid any negative effects of humidity and CO2.Results and discussion
The XRD patterns of the as-prepared pure MoSe2 and MoSe2@HCNF are shown in Fig. 1. From the XRD pattern of the as-prepared pure MoSe2, the diffraction peaks of (002), (004), (100), (103), (105), (110) and (108) planes indexing to the hexagonal MoSe2 phase (JCPDS 29-0914) can be observed at 13.3°, 26.2°, 31.5°, 37.8°, 46.5°, 56.0° and 66.5°, which suggest the successful synthesis of MoSe2. The as-prepared MoSe2@HCNF exhibits the same XRD pattern with pure MoSe2. The XRD pattern shows that the MoSe2 in both of the two samples maintained the 2H–MoSe2 structure.13 For MoSe2@HCNF, there should be a diffraction peak at 26.5°. However, the main peak of HCNF are masked by the overlap in the peak of (004) plane of MoSe2. This result indicates that the addition of HCNF can't affect the formation of MoSe2. And the Raman spectrum shown in Fig. 1b exhibits a much stronger A1g peak, corresponding to the out-of plane Mo–Se phonon mode, than the E12g peak, corresponding to the in-plane Mo–Se phonon mode. The specific intensity ration suggests the vertical growth of the nanosheets of MoSe2 on the surface of HCNF, which are made up of several stacked charge neutral layers consisted of three covalently bonded atomic sheets along the direction parallel to the surface of HCNF.13,16,18 | ||
| Fig. 1 (a) XRD patterns of the as-prepared pure MoSe2 and MoSe2@HCNF, (b) Raman spectrum of MoSe2@HCNF. | ||
The morphology of the pure MoSe2 and MoSe2@HCNF were characterized by SEM. From Fig. 2a, it can be clearly observed that the pure MoSe2 has a spherical-like morphology, which is further composed of MoSe2 nanosheets with graphene-like two-dimensional structure. And the MoSe2 nanosheets seriously agglomerate together as indicated in Fig. 2a. Comparing the SEM image of MoSe2@HCNF to HCNF shown in Fig. 2b, it can be found that the smooth surface of HCNF was completely covered by MoSe2 nanosheets after combining with MoSe2 and the morphology of the primary particles of MoSe2 have not been charged after combining with HCNF. This result demonstrates that the MoSe2@HCNF was successfully synthesized.
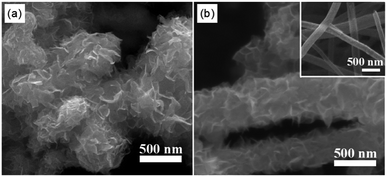 | ||
| Fig. 2 SEM images of the as-prepared (a) pure MoSe2 and (b) MoSe2@HCNF and the inset of (b) is SEM image of HCNF. | ||
In order to further examine the structure and morphology of the pure MoSe2 and MoSe2@HCNF, the TEM images of these two materials are shown in Fig. 3. The TEM images confirm that the basic units of these two materials are ultrathin MoSe2 nanosheets. The TEM images of MoSe2@HCNF (Fig. 3b) reveal that the vast majority of MoSe2 nanosheets uniformly grow along the orientation perpendicular to the surface of HCNF. To confirm MoSe2 and HCNF phases, the EDX analysis for C, Mo and Se were carried out. The C, Mo and Se elemental mapping images in Fig. 3e–h demonstrate that the Mo and Se elemental are evenly distributed over the outer layer of the fibrous of MoSe2/HCNF and overlap with the MoSe2 nanosheets in the hybrid, and the C elemental is evenly distributed over the core of the fibrous of MoSe2/HCNF and overlaps with the HCNF fiber substrate in the hybrid. From this result, it can be confirmed the presence of MoSe2 and HCNF phase in the MoSe2/HCNF hybrid material and the successful synthesis of the MoSe2/HCNF hybrid material. From the HRTEM images of the exposed edge sites of the MoSe2 nanosheets in pure MoSe2 (Fig. 3c) and MoSe2@HCNF (Fig. 3d), it can be discovered that the ultrathin nanosheets have an thickness of ∼5 nm and the distance of the adjacent lattice fringes in both of the two samples matches with the (002) plane lattice spacing of MoSe2 (JCPDS card no. 29-0914). This result demonstrate that the MoSe2 nanosheets is constituted by the stacking of several numbers of (002) planes. Therefore, it can be further confirmed that these exposed edge sites must be the edge sites of the (002) plane of MoSe2, which is reported to have excellent catalytic ability.13,15,18 The TEM image of pure MoSe2 (Fig. 3a) reveals that the center of the spherical-like particle is black and non-transparent, which illustrates that the agglomeration of the MoSe2 nanosheets in pure MoSe2 is serious. Oppositely, in the MoSe2@HCNF hybrids, the outline of HCNF can be clearly observed in the TEM images (Fig. 3b), which means the agglomeration of the two-dimensional sheet-like MoSe2 primary particles is greatly reduced. Therefore, the exposed edge sites of MoSe2@HCNF will be much more than that of pure MoSe2, which is conducive to the optimization of the catalytic effects of MoSe2. Furthermore, the complex of HCNF with MoSe2 nanosheets will contribute to the establishment of 3-D electric network in the MoSe2@HCNF hybrids, while the nanosheet structure is beneficial for the electronic conductivity in the monolayer of the (002) plane and the resistance is high across the different planes.13 And the improvement of electronic conductivity will also promote the catalytic activity.
 | ||
| Fig. 3 TEM images of the as-prepared (a and c) pure MoSe2 and (b and d) MoSe2@HCNF, and (e–h) elemental mapping images of MoSe2/HCNF hybrids. | ||
In order to gain straight insight to the electrocatalytic activity of the MoSe2@HCNF hybrids in Li–O2 batteries, the Super-P carbon black (SP) O2 electrodes with MoSe2@HCNF hybrids were assembled. The galvanostatic charge–discharge tests were carried out in O2 atmosphere at the voltage range of 2.0–4.4 V (vs. Li/Li+). For comparison, the SP electrodes with pure MoSe2 and the pure SP electrodes were also tested under the same conditions. The discharge and charge voltage curves obtained by discharging and recharging the Li–O2 batteries at the current density of 0.1 mA cm−2 are shown in Fig. 4. The pure SP electrode without catalyst shows a discharge capacity of 3572 mA h g−1carbon+catalyst and a much lower charge capacity of 2789 mA h g−1carbon+catalyst, which may be originated from the high charge voltage at ∼4.31 V. The SP electrode with pure MoSe2 exhibits a similar discharge voltage platform with the pure SP electrode at 2.61 V, but the charge voltage of the electrode is obviously decreased to 4.14 V after adding the pure MoSe2 and the charge capacity (3211 mA h g−1carbon+catalyst) is higher than that of pure SP electrode. However, the discharge capacity of the SP electrode with pure MoSe2, 3240 mA h g−1carbon+catalyst, is slightly less than that of pure SP electrode. These results signify that the MoSe2 have a certain catalytic ability, especially for the OER in the charge process of Li–O2 batteries, but the serious agglomeration of the MoSe2 nanosheets restricts the catalytic activity of the pure MoSe2. In contrast, the electrode with MoSe2@HCNF hybrids displays the best electrochemical performance in the three different electrodes. In spite of the similar discharge potential to the former two electrodes, the electrode with MoSe2@HCNF exhibits a relative large discharge capacity of 4487 mA h g−1carbon+catalyst. Moreover, the charging potential is significantly decreased to the lowest value of 4.01 V in the three electrodes, which results in the highest round-trip efficiency. And the charge capacity of the electrode with MoSe2@HCNF (4369 mA h g−1carbon+catalyst) is close to the discharge capacity, which manifest that the discharge products have almost been decomposed. These results prove that the MoSe2@HCNF possesses better catalytic activity than the pure MoSe2. Taking into account the content of the catalyst in the electrode and the carbon material used in the electrode are equal, we can reasonably speculate that the excellent catalytic ability of MoSe2@HCNF is related to the alleviation of the agglomeration, the increase of the edge sites and the enhancement of the electronic conductivity of the hybrids.
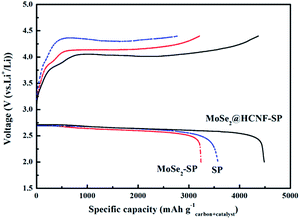 | ||
| Fig. 4 Discharge–charge curves of the SP electrode with MoSe2@HCNF, MoSe2 and without any catalyst between 2.0 and 4.4 V at 0.1 mA cm−2. | ||
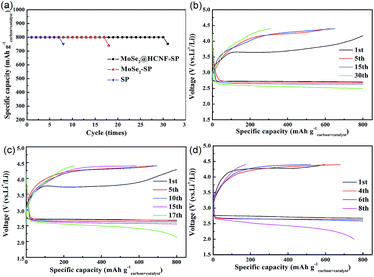
The cycle performance is one of the vital indexes that can measure the electrochemical performance of Li–O2 batteries. In the cyclic process, the accumulation of the small amount of undecomposed insulated discharge products and the insulated by-products originated from the decomposition of electrolyte during the cyclic process will gradually cover the positive electrode surface, and finally cause the end of the cycle due to the blocking of the pores or the passivation.20–22 Since the decomposition of the electrolyte usually become serious with the voltage rise and the decomposition of the discharge products is also affected by catalyst performance, the catalytic ability can also be measured by the cycling performance. In this study, the cycle test of the Li–O2 batteries with the three electrode with a capacity restriction of 800 mA h g−1carbon+catalyst at a current density of 0.1 mA cm−2 are performed, and the obtained cycle performance and the according voltage profiles are displayed in Fig. 5. The results show that the electrode with MoSe2@HCNF exhibits the best cycle performance up to 30 cycles and confirm the better catalytic activity of MoSe2@HCNF than pure MoSe2.
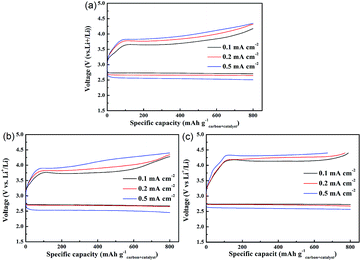
To measure the effects of current density on the discharge/charge voltages, the obtained discharge and charge curves of the three electrodes with a capacity restriction of 800 mA h g−1carbon+catalyst at different current densities are presented in Fig. 6. Although there are almost no differences in the discharge voltage plateaus of the three electrodes at the current density of 0.1 mA cm−2, the electrode with MoSe2@HCNF exhibits the lowest charge voltage of 3.69 V. Because of the higher ohmic losses caused by the higher current density, when the current density increases, it is reasonably to observe that the over-potentials of the three electrodes increased.23,24 Even though the discharge voltage plateaus of the three electrodes are similar to each other, the charge potentials of the electrode are obviously decreased by the addition of catalyst. And the electrode with MoSe2@HCNF still shows the lower charge voltage than the electrode with MoSe2. These results further demonstrate that, after combining MoSe2 with HCNF, MoSe2@HCNF presents an improved OER catalytic activity.
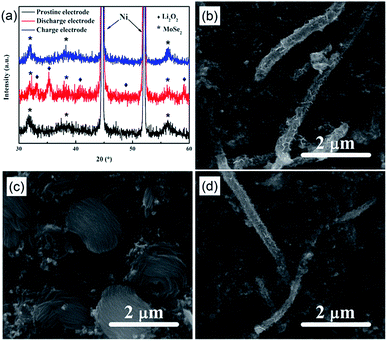
As shown in Fig. 7, the XRD patterns and SEM images of the pristine, discharged, and recharged air electrodes with MoSe2@HCNF hybrids were obtained. Form Fig. 7, it can be observed that the pristine cathode exhibits a porous surface before discharge (Fig. 7b). From the XRD pattern of the pristine electrode shown in Fig. 7a, only Ni peaks from current collector and MoSe2 peaks from MoSe2@HCNF hybrids can be observed. After discharge (Fig. 7c), the surface of the electrode was covered by massive insoluble discharged products with a toroidal shape, which is similar to the reported literatures,27–30 and the XRD analysis of the corresponding electrode shows that Li2O2 is the only detected crystalline product that appeared on the electrode after discharge (Fig. 7a).8,25,28,29 This result demonstrates that the solid discharge products deposited in the electrode is Li2O2. It can be observed from Fig. 7a that the toroidal solid discharge product on the electrode disappeared after recharge. And the XRD pattern for the recharged electrode shown in Fig. 7d reveals the peaks of Li2O2 are correspondingly disappeared. These results are similar to the results reported in the literatures about Li–O2 batteries we checked.26–29 These results indicate that the electrochemical reaction of Li–O2 batteries is based on the formation and decomposition of Li2O2 during charge and discharge and MoSe2@HCNF hybrids can catalyze Li2O2 reversible decomposition rather than electrolyte decomposition.
Conclusions
In summary, the MoSe2@HCNF hybrids are synthesized via a hydrothermal route. The as-prepared MoSe2@HCNF hybrids are used as the cathode catalyst of Li–O2 batteries for the first time. Governed by the more exposed edge sites originated from the successful growth of MoSe2 nanosheets on the surface of HCNF and the enhancement of the electronic conductivity of the composites resulted from the combination of HCNF, the MoSe2@HCNF hybrids show an improved catalytic activity with respect to pure MoSe2. And the MoSe2@HCNF hybrids exhibit superior catalytic activity for Li–O2 batteries, especially the OER catalytic activity for the charge process, and effectively reduced the charge over-potential. According to this study, it can be expected that metal selenides have a great potential to be conducted as the cathode catalyst for Li–O2 batteries.Notes and references
- R. S. Kalubarme, H. S. Jadhav, C.-N. Park, K.-N. Jung, K.-H. Shin and C.-J. Park, J. Mater. Chem. A, 2014, 2, 13024–13032 CAS.
- D. Oh, J. Qi, B. Han, G. Zhang, T. J. Carney, J. Ohmura, Y. Zhang, Y. Shao-Horn and A. M. Belcher, Nano Lett., 2014, 14, 4837–4845 CrossRef CAS PubMed.
- A. Riaz, K. N. Jung, W. Chang, K. H. Shin and J. W. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 17815–17822 CAS.
- C. K. Lee and Y. J. Park, Chem. Commun., 2015, 51, 1210–1213 RSC.
- F. Li, T. Zhang and H. Zhou, Energy Environ. Sci., 2013, 6, 1125–1141 CAS.
- Y. Shao, F. Ding, J. Xiao, J. Zhang, W. Xu, S. Park, J.-G. Zhang, Y. Wang and J. Liu, Making Li–Air Batteries Rechargeable: Material Challenges, Adv. Funct. Mater., 2013, 23, 987–1004 CrossRef CAS.
- W. Zhang, Y. Zeng, C. Xu, H. Tan, W. Liu, J. Zhu, N. Xiao, H. H. Hng, J. Ma, H. E. Hoster, R. Yazami and Q. Yan, RSC Adv., 2012, 2, 8508–8514 RSC.
- K. Zhang, L. Zhang, X. Chen, X. He, X. Wang, S. Dong, L. Gu, Z. Liu, C. Huang and G. Cui, ACS Appl. Mater. Interfaces, 2013, 5, 3677–3682 CAS.
- J. Zhang, P. Li, Z. Wang, J. Qiao, D. Rooney, W. Sun and K. Sun, J. Mater. Chem. A, 2015, 3, 1504–1510 CAS.
- W.-J. Kwak, K. C. Lau, C.-D. Shin, K. Amine, L. A. Curtiss and Y.-K. Sun, ACS Nano, 2015, 9, 4129–4137 CrossRef CAS PubMed.
- P. Zhang, X. Lu, Y. Huang, J. Deng, L. Zhang, F. Ding, Z. Su, G. Wei and O. G. Schmidt, J. Mater. Chem. A, 2015, 3, 14562–14566 CAS.
- M.-R. Gao, X. Cao, Q. Gao, Y.-F. Xu, Y.-R. Zheng, J. Jiang and S.-H. Yu, ACS Nano, 2014, 8, 3970–3978 CrossRef CAS PubMed.
- H. Tang, K. Dou, C.-C. Kaun, Q. Kuang and S. Yang, J. Mater. Chem. A, 2014, 2, 360–364 CAS.
- D. Kong, H. Wang, Z. Lu and Y. Cui, J. Am. Chem. Soc., 2014, 136, 4897–4900 CrossRef CAS PubMed.
- C. Xu, S. Peng, C. Tan, H. Ang, H. Tan, H. Zhang and Q. Yan, J. Mater. Chem. A, 2014, 2, 5597–5601 CAS.
- H. Wang, D. Kong, P. Johanes, J. J. Cha, G. Zheng, K. Yan, N. Liu and Y. Cui, Nano Lett., 2013, 13, 3426–3433 CrossRef CAS PubMed.
- C. Fan, Z. Wei, S. Yang and J. Li, RSC Adv., 2014, 4, 775–778 RSC.
- D. Kong, H. Wang, J. J. Cha, M. Pasta, K. J. Koski, J. Yao and Y. Cui, Nano Lett., 2013, 13, 1341–1347 CrossRef CAS PubMed.
- Z. Zhang, X. Yang, Y. Fu and K. Du, J. Power Sources, 2015, 296, 2–9 CrossRef CAS.
- X. Ren, S. S. Zhang, D. T. Tran and J. Read, J. Mater. Chem., 2011, 21, 10118–10125 RSC.
- C. Tran, X. Q. Yang and D. Y. Qu, J. Power Sources, 2010, 195, 2057–2063 CrossRef CAS.
- Y. Shao, S. Park, J. Xiao, J.-G. Zhang, Y. Wang and J. Liu, ACS Catal., 2012, 2, 844–857 CrossRef CAS.
- C. Xia, M. Waletzko, K. Peppler and J. Janek, J. Phys. Chem. C, 2013, 117, 19897–19904 CAS.
- C. Xia, C. L. Bender, B. Bergner, K. Peppler and J. Janek, Electrochem. Commun., 2013, 26, 93–96 CrossRef CAS.
- B. Sun, J. Zhang, P. Munroe, H.-J. Ahn and G. Wang, Electrochem. Commun., 2013, 31, 88–91 CrossRef CAS.
- C. Shang, S. Dong, P. Hu, J. Guan, D. Xiao, X. Chen, L. Zhang, L. Gu, G. Cui and L. Chen, Sci. Rep., 2015, 5, 8335 CrossRef CAS PubMed.
- R. R. Mitchell, B. M. Gallant, Y. Shao-Horn and C. V. Thompson, J. Phys. Chem. Lett., 2013, 4, 1060–1064 CrossRef CAS PubMed.
- D. Zhai, H.-H. Wang, J. Yang, K. C. Lau, K. Li, K. Amine and L. A. Curtiss, J. Am. Chem. Soc., 2013, 41, 15364–15372 CrossRef PubMed.
- J. Zeng, C. Francia, J. Amici, S. Bodoardo and N. Penazzi, J. Power Sources, 2014, 272, 1003–1009 CrossRef CAS.
- W. Fan, Z. Cui and X. Guo, J. Phys. Chem. C, 2013, 117, 2623–2627 CAS.
| This journal is © The Royal Society of Chemistry 2016 |