Chain-like Fe3O4@resorcinol-formaldehyde resins–Ag composite microstructures: facile construction and applications in antibacterial and catalytic fields†
Abstract
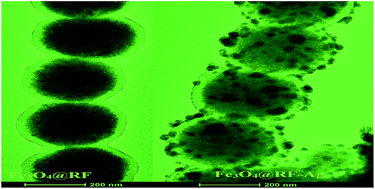
A facile two-step route is designed to synthesize chain-like Fe3O4@resorcinol-formaldehyde resins–Ag composite microstructures (denoted as Fe3O4@RF–Ag). Chain-like Fe3O4@RF microstructures are firstly obtained by the in situ polymerization of RF on Fe3O4 microspheres under the assistance of an external magnetic field; and then, Ag nanoparticles are strewn on the surface of the RF via in situ reduction by hydrazine hydrate. The as-obtained chain-like Fe3O4@RF–Ag microstructures are characterized by powder X-ray diffraction (XRD), scanning electron microscopy (SEM), (high-resolution) transmission electron microscopy (HRTEM/TEM) and energy dispersive spectrometry (EDS). Experiments indicate that the as-prepared chain-like Fe3O4@RF–Ag microstructures can not only serve as outstanding antibacterial agents against bacteria including Staphylococcus aureus and Escherichia coli, but also as highly efficient catalysts for the hydrogenation of various compounds, including 4-nitrophenol, methylene blue and Rhodamine B. Also, the as-prepared chain-like Fe3O4@RF–Ag microstructures can be conveniently regenerated under extra magnetic field due to the presence of Fe3O4 and they show good cyclic stability, which is important for practical applications.

 Please wait while we load your content...
Please wait while we load your content...