Enhanced antibiotic removal by the addition of bamboo charcoal during pig manure composting
Abstract
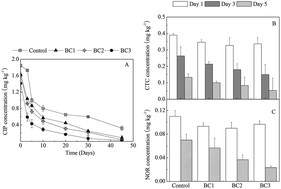
While composting is generally an effective way to minimize the adverse environmental impacts of manure prior to land application, some issues may also be introduced during compositing, such as the increased presence of recalcitrant antibiotic residues from feed additives. This study suggested that the addition of bamboo charcoal (BC) during pig manure composting was beneficial for the removal of three antibiotics (ciprofloxacin, chlorotetracycline, and norfloxacin). Addition of 9% (w/w) BC decreased the concentration of ciprofloxacin residues by 98.9% (from 1.85 to 0.02 mg kg−1 dry weight) in 45 days, and decreased the content of norfloxacin and chlorotetracycline below detection limits in less than 5 and 10 days, respectively. In comparison, without added BC, ciprofloxacin levels were decreased by only 82.7% (from 1.85 to 0.32 mg kg−1 dry weight) after 45 days of composting. This indicated that BC could enhance the removal of antibiotics during manure composting, thus reducing the risk of antibiotic residue runoff when composts are used in agriculture.

 Please wait while we load your content...
Please wait while we load your content...