Carbonyl scavenging and chemical chaperon like function of essential amino acids attenuates non-enzymatic glycation of albumin†
Saurabh Awasthi and
N. T. Saraswathi*
Molecular Biophysics Lab., School of Chemical and Biotechnology, SASTRA University, Thanjavur-613401, Tamilnadu, India. E-mail: saras@scbt.sastra.edu
First published on 25th February 2016
Abstract
Besides being fundamental in the development of severe complications such as retinopathy, neuropathy and kidney disorders, non-enzymatic glycation also modulates the structure and function of many proteins such as albumin hemoglobin and collagen etc. Here we report the inhibitory activity of essential amino acids (EAA) against the formation of early and advanced glycation end products based on fluorescence, circular dichroism and molecular interaction studies. Assays were carried out to determine the formation of fructosamine, inhibition of fluorescent AGEs, protein bound carbonyl content, lysine modification and effect on glycation induced fibrillation based on thioflavin T (ThT) binding and confocal imaging. The protective effect of EAA on albumin conformation was determined based on the circular dichroism analysis. EAA exhibited differential effects on the non-enzymatic glycation induced structural modification of albumin. In addition to this, EEA also showed potent inhibitory activity towards in vitro goat lens protein. Tryptophan, phenylalanine and lysine showed the highest and valine showed the lowest antiglycation activity. The molecular interaction pattern of EAA as determined by computational methods revealed their affinity, binding site and the residues involved in binding.
Introduction
Chronic hyperglycemia and the development of oxidative stress are fundamental in the development and progression of severe complications in diabetes.1,2 Non-enzymatic glycation is a slow multistep reaction which involves the reaction of free amino groups in proteins, nucleic acids and lipids with glucose or its metabolites giving rise to the formation of early glycation products also called Amadori products. Amadori products on oxidative modification give rise to the formation highly heterogeneous and reactive advanced glycation end products (AGEs).3–5 Formation and accumulation of AGEs are fundamental to the development of micro and macro-vascular complications,6 cataract,7 neurological complications2 and nephropathy8 etc. Several molecular approaches have been considered for inhibiting AGEs formation, and such diversity is due to the complex reactions leading to the AGE formation, which involves different pathways, precursors, intermediates and end products.9 The molecular strategies to combat the formation and accumulation of AGEs will need consideration of different approach in order to reduce the AGEs level in body such as; (a) early glycation inhibition (b) advanced glycation inhibition (c) carbonyl quenching (d) masking lysine residues and (e) inhibition of glycation induced aggregation.Albumin is the most abundant plasma protein in human serum which serves as a carrier for many endogenous and exogenous ligands. Crystal structure of albumin showed that it consists of three domains (I, II, III) each divided into two sub-domains (A and B). Besides being the major drug carrier, albumin also exhibits high affinity for fatty acids and contains seven fatty acid binding sites. The two major drug binding sites of albumin, also called as Sudlow's site I and II can bind with range of drugs and thus play crucial role in their distribution in body. Due to the direct exposure to the fluctuations in glycemic level, albumin is the highly susceptible protein for non-enzymatic glycation.10 In addition to this, albumin contains 59 lysine residues out of which 34 have been identified to be modified due to glycation. Glycation is known to induce structural changes and amyloid like fibrillation of albumin.11 Glycated albumin exhibits impaired functions such as drug binding, antioxidant activity, and esterase activity.
The progressive changes in lens protein glycation leading to the formation of high molecular weight aggregates have been identified to be fundamental in the development of diabetic cataract.12,13 Alpha-crystallin is a major lens protein which serves the structural function by facilitating the maintenance of the refractive index of the eye lens. In addition to its structural role, crystallin also exhibits chaperon like function which is helpful in preventing protein aggregation and cataractogenesis.14 Several natural and synthetic compounds which have been proposed as AGE inhibitors are either limited to the in vitro studies or have been associated with undesirable complications.15,16 Considering the side effects associated with the synthetic compounds there is a need for an alternative approach to preventing non-enzymatic glycation.
There is a need to adopt a healthy life style which lowers AGEs intake in the form of diet, minimize exposure to smoke, oxidants, radiation, and obesity. With this motivation, we aimed to determine the inhibitory potential of edible greens, nutraceuticals, and essential amino acids against formation and accumulation of AGEs.17,18 Amino acids play a key role in human nutrition and maintenance of health. Essential amino acids cannot be synthesized by the body and thus need to be supplied form of diet.19 There is a growing interest in biochemistry and physiology of amino acids in health and nutrition of humans.20 Recently amino acids have been proposed to be classified in to three classes namely; non-essential amino acids, which are biosynthesized by the body itself, essential amino acids; which body cannot synthesize and need to supplied in diet, and third new class known as functional amino acids which play role in signalling pathways, growth and metabolic diseases.21,22
The present study was aimed to demonstrate the inhibitory potential of nine essential amino acids against the formation of early and advanced glycation end products and their protective effects towards glycation induced structural modification of albumin. We also determined the antiglycation activity of amino acids towards goat lens protein to determine their protective effects in the prevention of cataractogenesis. The experimental studies involved the determination of fructosamine formation (early glycation product), fluorescent AGEs inhibition, protein bound carbonyl content, modification of lysine side chain, effect of amino acids on the conformation of BSA using circular dichroism (CD), and thioflavin T binding to study fibrillation inhibition. The molecular interaction pattern of all nine essential amino acids was determined based on computation approach.
Experimental
Chemicals
Bovine serum albumin (BSA), histidine (HIS), isoleucine (ILE), leucine (LEU), lysine (LYS), methionine (MET), threonine (THR), phenylalanine (PHE), tryptophan (TRP), valine (VAL), methylglyoxal (MG), aminoguanidine (AG), 2,4,6-trinitrobenzenesulfonic acid (TNBSA), and thioflavin T (ThT) were purchased from Sigma, USA. Di-nitro phenyl hydrazine (DNPH), guanidine hydrochloride and nitro blue tetrazolium (NBT) were obtained from SRL, India. All other chemicals of AR grade were purchased from Merck, India.In vitro glycation of BSA
BSA was glycated in vitro using moderate concentrations of glucose and methylglyoxal under highly sterile conditions. Briefly, 10 mg ml−1 of BSA was incubated in dark with glucose (25 mM) and methylglyoxal (10 mM) for the period of 30 days. A pre-incubation of 10 min was carried out with the test compound before the addition of glucose/methylglyoxal. Control samples include BSA alone, BSA + glucose and BSA + methylglyoxal. All glycated protein samples were extensively dialyzed and assays were carried out.Determination of fructosamine adduct
Fructosamine assay was used to determine the level of early glycation product formed as described by Baker et al., (1994)23 with slight modifications. Briefly, the glycated protein sample (1 mg ml−1) was incubated with NBT (150 μM) reagent prepared in sodium carbonate buffer for 30 min. The absorbance was recorded at 530 nm using Thermo Scientific Evolution-201 spectrophotometer. AG was used as a control.Inhibition of fluorescent AGEs
The estimations for AGEs fluorescence were carried out for glycated protein (10 mg ml−1) samples using Jasco-FP8200 spectrofluorimeter. The excitation (λex) and emission (λem) wavelengths for different AGEs as shown in Table 1, have been also given in our earlier work.24 Results were expressed in the form of fluorescent intensity of AGEs.Determination of carbonyl content
Protein bound carbonyl groups in glycated protein sample were determined using DNPH.28 The carbonyl content was expressed as nmol carbonyl mg−1 protein based on the extinction coefficient for DNPH (ε = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1).
000 M−1 cm−1).
TNBSA assay to determine lysine modification
Quantification of lysine modification was carried out by using TNBSA assay.29 Briefly, 500 μl of glycated BSA (1 mg ml−1) sample was incubated with 250 μl of 0.01% TNBSA for 2 hours at room temperature. After incubation 250 μl of 10% SDS and 1 N HCl was added and absorbance was read at 335 nm using Thermo Scientific Evolution-201 spectrophotometer. TNBSA was prepared in 100 mM sodium bicarbonate buffer (pH 8.5) and native BSA was used as control. Results were presented as the percent lysine modification.Thioflavin T fluorescence assay
To determine of inhibitory activity of EAA for β aggregation, ThT, a marker for amyloid cross β structure was used. Glycated samples and positive control (100 μL) were incubated with 32 μM ThT for 1 h at room temperature.30 Fluorescence was measured at λex and λem wavelengths of 435 and 485 nm (slit, 10 nm) respectively with correction for background signals without ThT. The results were expressed as % inhibition, calculated by the formula-inhibition% = [(F0 − FEAA)/F0] × 100, where F0 is the florescence of the control and FEAA is the florescence of the glycated albumin samples co-incubated with essential amino acids.Circular dichroism
CD spectra of BSA (1 μM) was measured using Jasco J-715 Circular Dichroism Spectropolarimeter at room temperature. A quartz cell of 1 mm path length was used for far-UV (195–260 nm) measurements with 1 nm bandwidth and the scan speed was set at 100 nm min−1. All spectra were derived from the average of three repeated scans for every sample.Inhibition of lens protein glycation in vitro
The total lens protein (TLP) was obtained from goat lenses as per the previous method.31 Briefly, goat lenses were homogenized in phosphate buffer saline (pH 7.4) and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for a duration of 30 min at 4 °C. Supernatant (TLP) was in vitro glycation reaction using glucose and used for determination of AGEs formation. The reaction mixture contained TLP, 0.2 M phosphate buffer, pH 7.4, glucose (25 mM), EAA (500 μM), and 0.01% sodium azide. Following the incubation period of three weeks, the formation of fluorescence AGEs was determined with excitation and emission wavelength of 330 and 440 nm using Jasco-FP8200 spectrofluorimeter.
000 rpm for a duration of 30 min at 4 °C. Supernatant (TLP) was in vitro glycation reaction using glucose and used for determination of AGEs formation. The reaction mixture contained TLP, 0.2 M phosphate buffer, pH 7.4, glucose (25 mM), EAA (500 μM), and 0.01% sodium azide. Following the incubation period of three weeks, the formation of fluorescence AGEs was determined with excitation and emission wavelength of 330 and 440 nm using Jasco-FP8200 spectrofluorimeter.
Computational method
The computational studies involving molecular docking simulations were carried out to determine the interactions of different EAA with BSA. AutoDock Vina 4.0 (ref. 32) was used to carry out docking studies. The structure of proteins and ligands and were retrieved from the Protein Data Bank (PDB) and PubChem chemical compounds database respectively. Both protein and ligands were preprocessed using AutoDcok tools, which involved the addition of Kollman charges, polar hydrogen and energy minimization. A comparative analysis was carried out for the docking score of EAA and binding pattern was further analyzed using Pymol.Results and discussion
In our approach towards determining the antiglycation activity of essential amino acids, the present study employed the in vitro glycation model of BSA. All assays were carried out using different concentrations (50, 100, and 500 μM) of EAA. Small molecules with amino groups such as aminoguanidine, metformin etc. have been found to exhibit potent antiglycation activities.33 The antiglycation activities of only three amino acids i.e. D-lysine34 L-arginine35 and recently L-cystine36 have been reported and the role of essential amino acids which are the key part of nutrition and human health remained yet to be determined.Effect of EAA on early glycation product (fructosamine)
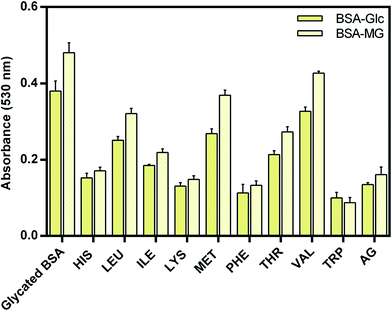
Fructosamine is also a marker for glycemic level in diagnosis of diabetes patients. Estimations of fructosamine formation have been extensively used in order to determine the level of early glycation product37,38 which is also called Amadori product. NBT assay was carried out to determine the inhibitory potential of EAA against at the early stage of glycation and results are presents in Fig. 1. NBT reacts with the fructosamine and gives rise to the blue color product, which exhibits absorbance at 530 nm. It was observed that EAA reduces early glycation and formation of Amadori product, which was evident from the reduced absorbance at 530 nm.From results, it was evident that EAA exhibited differential inhibitory activity towards early glycation of BSA. Tryptophan showed highest inhibitory activity followed by PHE, LYS and HIS and other amino acids. Inhibition of Maillard reaction at the early stage of glycation could be helpful in avoiding the formation of more reactive, and toxic advanced glycation end products.
Effect of EAA on advanced glycation product (AGEs)
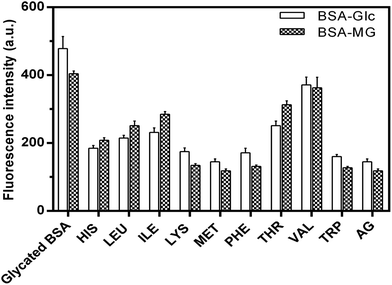
Advanced glycation end products (AGEs) are highly reactive and fundamental in the development of diabetic complications such as nephrological disorders, Alzheimer's disease and skin aging. AGEs are characterized by their specific properties such as fluorescence, cross-linking and activation of signaling cascades for inflammatory pathways.39 Fluorescence spectroscopy was used to determine the inhibitory activity of EAA against the formation of fluorescent AGEs. Results are presented in the Fig. 2 in the form of the fluorescence intensity for different AGEs in presence and absence of EAA.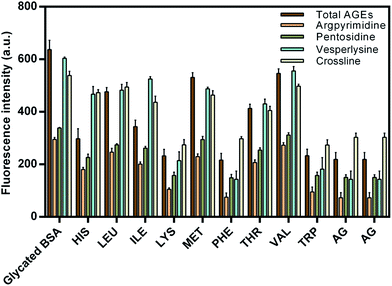 | ||
| Fig. 2 Effect of essential amino acids on advanced glycation end products (AGEs). Results are means ± standard deviations of three different assays. | ||
In addition to the total AGEs the fluorescence intensity estimations were also carried out for the four specific AGEs (argpyrimidine, pentosidine, vesperlysine and crossline). The main reason for considering these four specific AGEs was their role in the development of severe complications and their well defined fluorescence properties. It was seen that when compared to the negative control (glycated BSA), the presence of EAA showed comparatively reduced fluorescence intensity. TRP showed the least fluorescence intensity and thus highest inhibitory activity when compared to the other EAA. The maximum AGEs inhibition achieved was found to be up to 80%. Also the AGEs inhibitory activity of PHE, TRP and LYS was found to be greater or similar to that of aminoguanidine, which was used a positive control.
Effect of EAA on bound carbonyl content and lysine modification
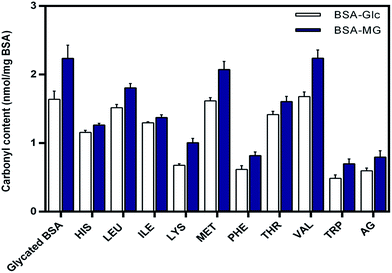
Both synthetic and natural compound with high scavenging potential for carbonyl compounds have been found to be potent inhibitor of glycation.18,40 DNPH assay was carried out in order to determine the level of protein bound carbonyl groups and results are presented in the Fig. 3. From results it was evident that there was a reduction in level of carbonyl content in the presence of EAA. The carbonyl scavenging potential of TRP and PHE was found to be similar to that of aminoguanidine. These observations suggest the effective role of EAA as a potent scavenger of carbonyl compounds, which serve as an intermediated of AGEs.Lysine residues in proteins have been found to be most susceptible for the non-enzymatic glycation modification giving rise to the formation of range of AGEs such as carboxymethyllysine (CML), carboxyethyllysine (CEL) and vesperlysine (VESP) etc. Mass spectroscopic studies in the recent have shown that out of the 59 lysine residues in BSA 34 serve as a site of glycation.41 Thus, determining the level of lysine modification could provide the information about the protective effects of essential amino acids towards non-enzymatic glycation of BSA. TNBSA assay was used for the quantification of lysine modification and results for are presented in the Fig. 4.
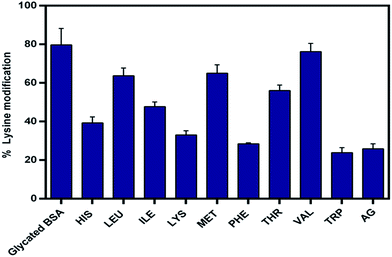 | ||
| Fig. 4 Effect of essential amino acids on lysine modification due to glycation. Results are means ± standard deviations of three different assays. | ||
The extent of lysine modification was found to be as high as 80% in case glycated BSA control. As shown in the figure the presence of EAA showed comparatively low level of lysine modification. In case of TRP, lysine modification was reduced to be as low as 23%, which was comparatively better than aminoguanidine (25%). Other than TRP, PHE, LYS and HIS also showed a significant decrease in the lysine modification. Valine showed almost no effect with least reduction in lysine modification due to glycation. These observations were indicative of protective effect of EAA towards non-enzymatic glycation of lysine.
Effect of EAA on fibrillation of albumin
Glycation modulates structure of albumin and induces amyloid like fibrillation.11 Protein fibrillation and accumulation of amyloid like aggregates is a key step in the development of several neurological disorders such as Parkinson's disease, Prion's disease etc.42 In order to determine the inhibition of glycation induced aggregation and fibrillation of BSA, ThT binding assay was carried out and results are presented in the Fig. 5. ThT specifically binds to the amyloid fibrils and shows enhanced fluorescence intensity at 485 nm. From the results it was evident that amino acid restrain glycation induced aggregation and fibrillation of BSA.Out of the all EAA, TRP, MET, LYS and PHE showed maximum reduction in the fluorescence intensity when compared to the glycated BSA control. VAL and THR showed least reduction in the ThT fluorescence when compared to the other essential amino acids. Inhibitors of protein aggregation and fibrillation could play a preventive role in the development of severe neurological disorders such as Alzheimer's disease. These results were indicative of the anti-fibrillation potential of essential amino acids.
Effect of EAA on conformation of albumin
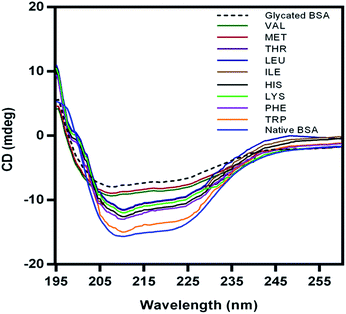
Non-enzymatic glycation induces conformational transition in albumin leading to the secondary structural changes from helical to beta sheeted structure. CD analysis can provide useful information about glycation mediated conformational changes in proteins. The protective effect of essential amino acids for the structure of BSA was determined based on circular dichroism (CD) analysis and CD spectra are presented in Fig. 6.Glycated BSA showed beta sheeted conformation when compared to the native helical BSA. The protective effect of EAA for glycation induced conformational changes in BSA was evident from the CD spectra of BSA glycated in the presence of amino acids. Compared to the other EAA, TRP showed highest activity and CD spectra similar to native BSA. Even earlier studies have showed amino acids such as L-arginine and L-lysine exhibits chemical chaperon like function and stabilize proteins against aggregation.43,44 Such protective effect of EAA towards the native conformation of BSA could also helpful in the prevention of glycation induced amyloid. And also this protective effect of EAA for native conformation of BSA could be attributed to their chemical chaperon like activity.
Molecular interaction of EAA with BSA
Docking studies were used in order to get insights about the molecular interaction pattern of all nine essential amino acids with BSA. Masking of free amino groups in proteins has been proposed to be one of the mechanisms for the antiglycation effects of small molecule inhibitors of glycation.45 The binding energies (kcal mol−1) as determined from the molecular docking studies of all EAA and BSA complex are given in Fig. 7. It can be seen that PHE (−4.9), TRP (−4.8) and THR (−4.4) showed highest affinity for BSA when compared to the other amino acids. The affinity of EAA with BSA was mainly determined by hydrogen bonding interaction.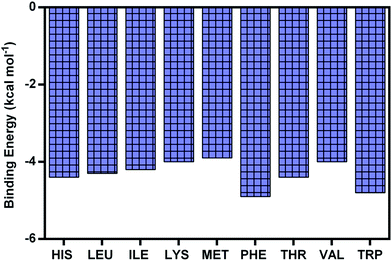 | ||
| Fig. 7 The binding energy of all nine essential amino acids with albumin as determined from molecular docking approach. | ||
Furthermore, observations for the molecular interaction of EAA-BSA complexes were carried out and the best poses obtained from the molecule docking simulations were presented in the Fig. 8. The residues interacting with the EAA are shown in figure and hydrogen bonds (H-bond) are represented as yellow dashed line. The distance of H-bonds formed by EAA is given in angstrom (Å). From the observations of docked structure it was evident that EAA bind with BSA by mainly interacting with both lysine and arginine residues. Interestingly there was no H-bonding interaction seen in case of valine, which also showed least antiglycation activity.
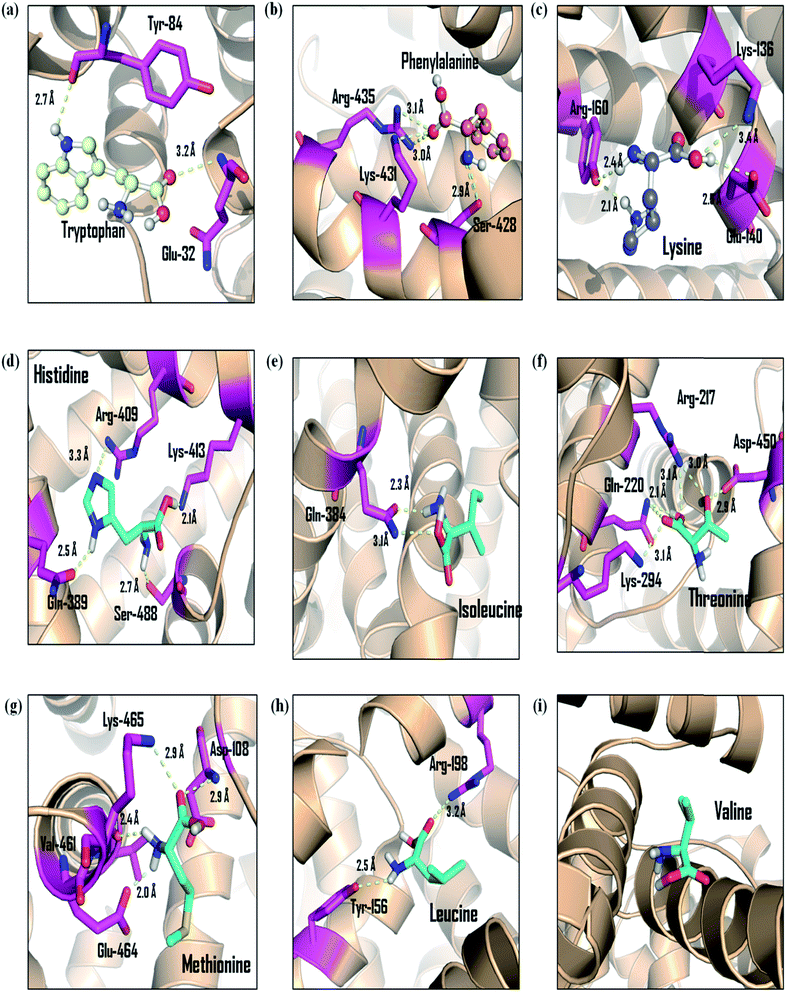 | ||
| Fig. 8 The molecular interaction pattern of essential amino acids with albumin as determined from molecular modeling studies. Hydrogen bonds are represented by the yellow color dashed lines. | ||
The results from the molecular interaction studied of EAA were in good agreement with the other results from the assays to determine the antiglycation activity. And based on these observations this can be hypothesized that the masking of lysine and arginine residues is possibly one of the mechanism responsible for the potent antiglycation activity of EAA.
Effect of EAA on in vitro glycation of lens proteins
Glycation of lens proteins has been established as one of the primary cause of cataractogenesis and development of blindness with the process of aging.12,13,46 In an attempt to look into the antiglycation potential of EAA for lens proteins, goat lens crystallin was used for in vitro glycation assays. The antiglycation potential of EAA for lens crystallin was determined based on the estimation of AGEs fluorescence and results are presented in the Fig. 9.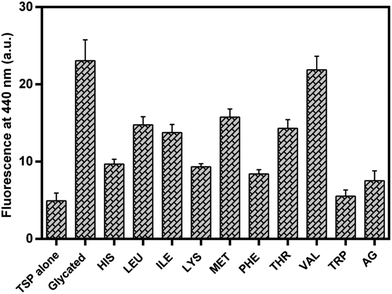 | ||
| Fig. 9 Effect of essential amino acids on lens crystallin glycation. Results are means ± standard deviations of three different assays. | ||
It was observed that except valine all other EAA exhibited reduced AGEs fluorescence when compared to the glycated TLP. Tryptophan showed least AGEs fluorescence when compared to the other EAA. Phe, Lys and His also showed about 50% reduction in the AGEs fluorescence intensity.
Docking studies were also carried out for the lens crystallin and binding pattern of TRP, PHE, LYS and VAL is shown in Fig. 10. It can be seen that TRP, PHE and LYS interact with lysine and arginine residues in lens protein. On the other hand, such interaction was not observed in the case of valine.
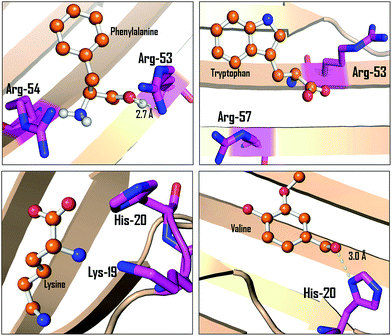 | ||
| Fig. 10 The molecular interaction pattern of phenylalanine, tryptophan, lysine and valine with alpha-crystallin as determined from molecular docking studies. | ||
In a recent report by Zhao et al. (2015)47 lanosterol was found to exhibits potent anti-cataract activity by preventing aggregation of lens crystallin. Our results suggest that the antiglycation activity EAA could also play a preventive role in the towards lens protein glycation.
Conclusions
In summary, this is a first demonstration of the protective effects of essential amino acids towards the non-enzymatic glycation of albumin. The key observations of this study were (1) except valine all EAA showed antiglycation activity (2) TRP and PHE showed the highest inhibitory activity (3) EAA also exhibited protective effects towards glycation of goat lens crystallin and (4) The antiglycation activity of EAA can be mainly attributed to their carbonyl scavenging, chemical chaperon like activity and masking of lysine and arginine residues. Strategies which could reduce formation and accumulation of AGEs can be helpful to slowdown the tissue aging and improve the lifespan.Acknowledgements
Authors thank SASTRA University for providing the financial support (TRR Fund) and infrastructure to carry out the research work.References
- S. Yamagishi, S. Maeda, T. Matsui, S. Ueda, K. Fukami and S. Okuda, Biochim. Biophys. Acta, 2012, 1820, 663–671 CrossRef CAS PubMed.
- G. Münch, B. Westcott, T. Menini and A. Gugliucci, Amino Acids, 2012, 42, 1221–1236 CrossRef PubMed.
- V. P. Singh, A. Bali, N. Singh and A. S. Jaggi, Korean J. Physiol. Pharmacol., 2014, 18, 1–14 CrossRef CAS PubMed.
- C. Ott, K. Jacobs, E. Haucke, A. Navarrete Santos, T. Grune and A. Simm, Redox Biol., 2014, 9, 411–429 CrossRef PubMed.
- R. Milne and S. Brownstein, Amino Acids, 2013, 44, 1397–1407 CrossRef CAS PubMed.
- M. Peppa and S. A. Raptis, Curr. Diabetes Rev., 2008, 4, 92–100 CrossRef CAS PubMed.
- M. Smuda, C. Henning, C. T. Raghavan, K. Johar, A. R. Vasavada, R. H. Nagaraj and M. A. Glomb, Biochemistry, 2015, 54, 2500–2507 CrossRef CAS PubMed.
- M. C. Thomas, J. M. Forbes and M. E. Cooper, Am. J. Therapeut., 2005, 12, 562–572 CrossRef.
- G. Aldini, G. Vistoli, M. Stefek, N. Chondrogianni, T. Grune, J. Sereikaite, I. Sadowska-Bartosz and G. Bartosz, Free Radical Res., 2013, 1, 93–137 CrossRef PubMed.
- A. Raghav and J. Ahmad, Diabetes & Metabolic Syndrome: Clinical Research & Reviews, 2014, 8, 245–251 Search PubMed.
- B. Bouma, L. M. Kroon-Batenburg, Y. P. Wu, B. Brünjes, G. Posthuma, O. Kranenburg, P. G. de Groot, E. E. Voest and M. F. Gebbink, J. Biol. Chem., 2003, 278, 41810–41819 CrossRef CAS PubMed.
- Z. Hashim and S. Zarina, Age, 2011, 33, 377–384 CrossRef CAS PubMed.
- R. E. Perry, M. S. Swamy and E. C. Abraham, Exp. Eye Res., 1987, 44, 269–282 CrossRef CAS PubMed.
- J. Horwitz, M. P. Bova, L. L. Ding, D. A. Haley and P. L. Stewart, Eye, 1999, 13, 403–408 CrossRef PubMed.
- X. Peng, J. Ma, F. Chen and M. Wang, Food Funct., 2011, 2, 289–301 CAS.
- S. Rahbar and J. L. Figarola, Arch. Biochem. Biophys., 2003, 419, 63–79 CrossRef CAS PubMed.
- G. Prasanna and N. T. Saraswathi, Planta Med., 2013, 79, PN84 Search PubMed.
- G. Prasanna and N. T. Saraswathi, J. Biomol. Struct. Dyn., 2015 DOI:10.1080/07391102.2015.1060160.
- G. Wu, Amino Acids, 2009, 37, 1–17 CrossRef CAS PubMed.
- E. Volpi, H. Kobayashi, M. Sheffield-Moore, B. Mittendorfer and R. R. Wolfe, Am. J. Clin. Nutr., 2003, 78, 250–258 CAS.
- G. Wu, Amino Acids, 2013, 45, 407–411 CrossRef CAS PubMed.
- Y. Hou, Y. Yin and G. Wu, Exp. Biol. Med., 2015, 240, 997–1007 CrossRef CAS PubMed.
- J. R. Baker, D. V. Zyzak, S. R. Thorpe and J. W. Baynes, Clin. Chem., 1994, 40, 1950–1955 CAS.
- S. Awasthi and N. T. Saraswathi, RSC Adv., 2015, 5, 87660–87666 RSC.
- P. J. Thornalley and N. Rabbani, Biochim. Biophys. Acta, 2014, 1840, 818–829 CrossRef CAS PubMed.
- R. Meerwaldt, T. Links, R. Graaff, S. R. Thorpe, J. W. Baynes, J. Hartog, R. Gans and A. Smit, Ann. N. Y. Acad. Sci., 2005, 1043, 290–298 CrossRef CAS PubMed.
- M. Bohlooli, A. A. Moosavi-Movahedi, F. Taghavi, P. Maghami, A. A. Saboury, Z. Moosavi-Movahedi, M. Farhadi, J. Hong, N. Sheibani and M. Habibi-Rezaei, Int. J. Biol. Macromol., 2013, 62, 358–364 CrossRef CAS PubMed.
- R. L. Levine, D. Garland, C. N. Oliver, A. Amici, I. Climent, A. G. Lenz, B. W. Ahn, S. Shaltiel and E. R. Stadtman, Methods Enzymol., 1990, 186, 464–478 CAS.
- P. Cayot and G. Tainturier, Anal. Biochem., 1997, 249, 184–200 CrossRef CAS PubMed.
- H. LeVine 3rd, Methods Enzymol., 1999, 309, 274–284 CAS.
- P. A. Kumar, M. S. Kumar and G. B. Reddy, Biochem. J., 2007, 408, 251–258 CrossRef CAS PubMed.
- O. Trott and A. J. Olson, J. Comput. Chem., 2010, 31, 455–461 CAS.
- P. J. Thornalley, Arch. Biochem. Biophys., 2003, 419, 31–40 CrossRef CAS PubMed.
- M. Sensi, F. Pricci, M. G. De Rossi, S. Morano and U. Di Marlo, Clin. Chem., 1989, 35, 384–387 CAS.
- D. A. Servetnick, D. Bryant, K. J. Wells-Knecht and P. L. Wiesenfeld, Amino Acids, 1996, 11, 69–81 CrossRef CAS PubMed.
- S. Mahdavifard, S. Z. Bathaie, M. Nakhjavani and H. Heidarzadeh, Food Res. Int., 2014, 62, 909–916 CrossRef CAS.
- V. Jakus, K. Bauerova, D. Michalkova and J. Carsky, Bratisl. Lek. Listy, 2000, 101, 484–489 CAS.
- V. M. Monnier, D. R. Sell and S. Genuth, Ann. N. Y. Acad. Sci., 2005, 1043, 567–581 CrossRef CAS PubMed.
- A. Schmitt, J. Schmitt, G. Münch and J. Gasic-Milencovic, Anal. Biochem., 2005, 338, 201–215 CrossRef CAS PubMed.
- H. Liu, H. Liu, W. Wang, C. Khoo, J. Taylor and L. Gu, Food Funct., 2011, 2, 475–482 CAS.
- J. Anguizola, R. Matsuda, O. S. Barnaby, K. S. Hoy, E. DeBolt, M. Koke and D. S. Hage, Clin. Chim. Acta, 2013, 425, 64–76 CrossRef CAS PubMed.
- D. Eisenberg and M. Jucker, Cell, 2012, 148, 1188–1203 CrossRef CAS PubMed.
- B. M. Baynes, D. I. Wang and B. L. Trout, Biochemistry, 2005, 44, 4919–4925 CrossRef CAS PubMed.
- A. Jafarnejad, S. Z. Bathaie, M. Nakhjavani, M. Z. Hassan and S. Banasadegh, Diabetes/Metab. Res. Rev., 2008, 24, 64–73 CrossRef CAS PubMed.
- M. M. Joglekar, S. N. Panaskar, A. D. Chougale, M. J. Kulkarni and A. U. Arvindekar, Mol. BioSyst., 2013, 9, 2463–2472 RSC.
- S. A. Kandarakis, C. Piperi, F. Topouzis and A. G. Papavassiliou, Prog. Retinal Eye Res., 2014, 42, 85–102 CrossRef CAS PubMed.
- L. Zhao, X. L. Chen, J. Zhu, Y. B. Xi, X. Yang and L. D. Hu, et al., Nature, 2015, 523, 607–611 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27460e |
| This journal is © The Royal Society of Chemistry 2016 |