The oxygen reduction reaction of ordered porous carbon-supported PtSn catalysts†
Chin-Tien Shena,
Kuan-Wen Wangb,
Chung-Jen Tseng*a,
Kan-Rong Leea and
Yu-Jui Hsueha
aDepartment of Mechanical Engineering, National Central University, No. 300, Jhongda Rd., Jhongli District, Taoyuan City 32001, Taiwan. E-mail: cjtseng@ncu.edu.tw; Fax: +886-3-4254501; Tel: +886-3-4267348
bInstitute of Materials Science and Engineering, National Central University, Taoyuan 32001, Taiwan
First published on 28th April 2016
Abstract
In this study, ordered porous carbon (OPC) prepared by a template method has been used as the catalyst support for cathodic oxygen reduction reaction (ORR) due to its large surface area and continuous structure. PtSn alloy nanoparticles with a size of 2.7 nm are deposited on OPC by using alcohol reduction method. The synergistic effect of Sn alloying and OPC support can not only modify the surface chemical states of Pt and Sn but also affect the d-band vacancy of Pt. The X-ray photoelectron spectroscopy and X-ray absorption near edge spectroscopy reveal that the oxidation of Pt is suppressed in PtSn/OPC, thus promoting the ORR performance before and after accelerated durability test.
1. Introduction
The development of highly effective and stable electrocatalysts toward the oxygen reduction reaction (ORR) is an important task for proton exchange membrane fuel cells (PEMFCs).1–6 By alloying with different metals, changing the morphologies, and using various supports, the ORR performance of Pt catalysts can be enhanced to different extents.7–11 The proposed volcano type behavior suggest that two factors, adsorption energies of oxygen containing species and surface coverage by blocking species, determine the ORR performance of Pt alloys.12 It has been reported that the ORR activity of Pt alloys is related to the oxophilicity in which Au alloying can prevent Pt from oxidation and stabilize the catalysts during long-term durability,13,14 suggesting the importance to modify the chemical states and electronic configurations of Pt.Moreover, changing the support is an alternative way to enhance the ORR performance of Pt/C. Yu et al.15 have applied porous carbon support to direct methanol fuel cells (DMFCs), and found its specific surface area is 2.5 times higher than conventional carbon black. The performance of a fuel cell with porous carbon support is found to be 15% higher than a cell with traditional carbon black. On the other hand, Ambrosio et al.16 have pointed out that although using porous carbon as catalyst support may reduce catalyst particle size and enhance the electrochemically active surface area, there is no obvious effect in improving ORR. They have suggested that electronic conductivity and structure of porous carbon are the main factors influencing the cell performance. Song et al.17 studied the effect of pore morphology on the catalyst activity in mesoporous carbon: OMC-CMK-3 (ordered mesoporous carbon). They found that highly ordered OMC-CMK-3 provided Pt nanoparticles with more electrochemically active Pt sites and a higher electrochemical surface area. In addition, Calvillo et al.18 showed a better electrocatalytic performance than commercial Pt/C black, possibly due to effective hydrogen diffusion to the active catalyst sites through the ordered porous structure of the support. Besides, ultrafine porous carbon fiber has been used as a novel compound carbon support (CCS) to support Pt catalyst (Pt/CCS).19 The performance of a single fuel cell catalyzed by Pt/CCS has shown 1.25 times higher power density than that catalyzed by Pt/C owing to the reduction of mass transform resistance.19 Furthermore, functional nanoporous carbon materials with the control of porosity, crystallinity, and morphology, surface structure, framework composition have been applied as supports for fuel cells.18 Compared to traditional carbon materials, porous carbon materials have the advantages of high surface area, accessible pore and highly graphitic structure, which are excellent materials for loading catalysts and accelerating mass and electron transportation.20
Therefore in this study, the ORR performance of Pt catalysts are promoted by Sn alloying and ordered porous carbon (OPC) support. PtSn catalysts have shown potential as effective catalysts towards ORR and ethanol oxidation reaction.5,21,22 PtSn catalysts and OPC have been prepared by alcohol-reduction process and template synthesis method, respectively. We have demonstrated that the effect of chemical states and electronic structure modification of Pt on its ORR performance by the characterization of X-ray photoelectron spectroscopy (XPS) and X-ray absorption near edge spectroscopy (XANES).
2. Experiment procedure
2.1. Preparation of OPC
In this work, silica (SiO2) particles were used as the template.15,23 Ethanol (C2H5OH), methanol (CH3OH), and de-ionized (DI) water were mixed in different volume ratios. The solution was stirred for 30 min. Ammonia water (NH4OH), and tetraethoxy silane (TEOS) was then added sequentially. The resulting solution was stirred, mixed for 6 hours, and then baked at 120 °C to obtain white silica powders. Phenolic resin powders were used as the carbon precursor, which were dissolved in ethanol first, and then mixed with silica powders. The mixture was baked at 125 °C in a vacuum oven for 12 hours first to remove residual water and organics, and then calcined at 800 °C under argon atmosphere for 12 hours. Finally, the silica template was removed by immersing in 48% hydrofluoric acid (HF) solution with stirring for 24 hours. After washing with DI water and alcohol, OPC can be obtained.242.2. Preparation of catalysts
2.3. Characterization
The morphologies of the OPC were observed by using a scanning electron microscopy (SEM, JEOL JSM-7401F). The surface areas were determined from N2 sorption/desorption using an accelerated surface area and porosimetry (ASAP 2010, Micromeritics) apparatus. Prior to the analysis, the samples were degassed under vacuum at 125 °C for 24 h. 0.1 g powders were then heated at 250 °C under vacuum for 2 hours until the degree of vacuum was less than 6 μm Hg. The specific surface area (SBET) was determined using BET (Brunauer–Emmett–Teller) model. The micropore volume, specific surface area of macropores, specific surface area of mesopores and specific surface area of micropores were calculated using the Harkins–Jura model (t-plot analysis). All textural parameters are summarized in Table 2. The structures of the catalysts were determined by X-ray diffraction (XRD) using a Siemens D-5000 with a Cu Kα radiation source. The morphology of the catalysts was analyzed by high resolution transmission microscopy (HRTEM, JEOL JEM-2100). The XPS (Thermo VG Scientific Sigma Probe) using a monochromatic X-ray source (Al Kα) at a voltage of 20 kV and a current of 30 mA was executed to identify the surface chemical states of the catalysts. The atomic ratios of Pt/Sn were examined by energy dispersive spectrometer (EDS) analysis.The X-ray absorption spectroscopy (XAS) spectra of catalysts were obtained in fluorescence mode at the BL17C beamlines at National Synchrotron Radiation Research Center (NSRRC), Taiwan. A Si monochromator was employed to adequately select the energy with a resolution ΔE/E better than 10−4 at Pt LII (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 273 eV) and LIII-edges (11
273 eV) and LIII-edges (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 564 eV). The un-filled d-states (HTs) were obtained from the Pt LII and LIII white lines of XANES. The fractional changed in the number of d-band vacancies relative to the reference material (fd) could be estimated:25
564 eV). The un-filled d-states (HTs) were obtained from the Pt LII and LIII white lines of XANES. The fractional changed in the number of d-band vacancies relative to the reference material (fd) could be estimated:25
| fd = ΔA3 + 1.11ΔA2/(A3 + 1.11A2)r | (1) |
| ΔA2 = (A2s − A2r) and ΔA3 = (A3s − A3r), | (2) |
| HTs = (1 + fd)HTr | (3) |
Electrochemical measurements of the electrocatalysts were performed in a standard three-compartment electrochemical cell with reference and counter electrodes in separate compartments to the working electrode. The Ag/AgCl electrode and a Pt wire served as reference and counter electrodes, respectively. An amount of 5 mg of the catalysts were dispersed in 1 mL 2-propanol (IPA) and 5 μL 5% Nafion® solution for 30 min, obtaining a well-dispersed catalyst ink. The resulting ink was transferred to the glassy carbon disk (0.196 cm2) of the working electrode. The final loading of Pt on each electrode was ca. 9.5 μg for the Pt/C, 7.1 μg for the bimetallic PtSn/C and PtSn/OPC electrodes. Cyclic voltammetry (CV) and linear sweep voltammetry (LSV) were performed to study the catalytic activity. A rotating disk electrode (RDE) was used to measure the ORR activity of the catalysts in an oxygen-purged solution of 0.5 M H2SO4 by LSV. The rotational speed was set at 1600 rpm and scanning rate was 5 mV s−1. All potentials quoted were referred to the normal hydrogen electrode (NHE). During the measurements, a moderate O2 gas flow was kept above the electrolyte. Comparison of ORR activities for all catalysts within the mixed kinetic-diffusion region (current density at E = 0.85 V, Ik085). The Ik was calculated based on the following equation:
| Ik = IdI/Id − I | (4) |
On the other hand, the RDE measurements under various rotational speeds for the Pt/C, PtSn/C and PtSn/OPC are used to calculate the electron transfer numbers (n) during the ORR as shown in Fig. S5.† Fig. S5(d)† shows their corresponding Koutecky–Levich plot drawn against the inverse current density (j−1) as a function of the inverse square root of the rotation rate (ω−1/2). The Koutecky–Levich equation is
| 1/I = 1/Ik + 1/Id = 1/Ik + 1/βω1/2 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485 C mol−1, the bulk concentration of oxygen (C) equal to 1.13 × 10−6 mol cm−3, the diffusion coefficient for oxygen D equal to 1.8 × 10−5 cm2 s−1, the viscosity of electrolyte (ν) equal to 10.78 × 10−2 cm2 s−1 in 0.5 M H2SO4 solution have been used.26
485 C mol−1, the bulk concentration of oxygen (C) equal to 1.13 × 10−6 mol cm−3, the diffusion coefficient for oxygen D equal to 1.8 × 10−5 cm2 s−1, the viscosity of electrolyte (ν) equal to 10.78 × 10−2 cm2 s−1 in 0.5 M H2SO4 solution have been used.26
Chronoamperometry tests for the ORR at 0.6 V (vs. Ag/AgCl) were carried out for 1 h. The accelerated durability test (ADT) was performed to accelerate the degradation of the catalysts by continuously cycling the potential in 0.5 M H2SO4 for 1500 CV cycles. The scanning rate was 50 mV s−1. The electrochemical stability of the catalysts was studied by LSV after the ADT.
3. Results and discussion
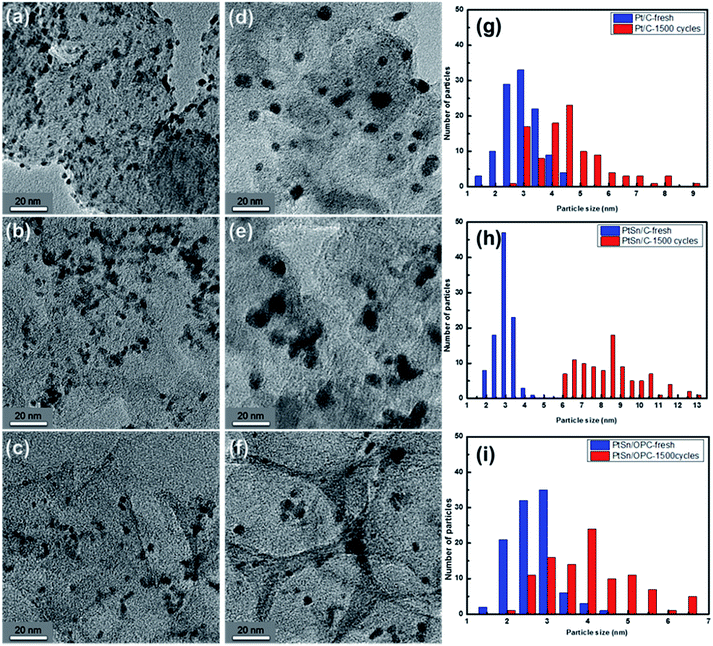
The size of silica particles can be controlled by adjusting the amount of TEOS, the alcohol concentration, pH value, or temperature.27 In this study, the particle size of silica is controlled by changing the volume ratio of ethanol to methanol. As shown in Fig. S1(a)† in ESI, uniform silica powders with high reproducibility and a size of 180 nm can be prepared. On the other hand, the porous structure and pore size of OPC depend on the shape and dimension of the template. Fig. S1(b)† shows the SEM images of the OPC. Some fragments and defects appear on the surface, possibly due to stirring during the preparation process. The pore size of this OPC sample is about 150 nm, slightly smaller than that of the silica particles, mainly due to shrinkage at high temperature. The specific surface area of OPC sample is measured by using ASAP. Based on the principle of BET,28 the specific surface area of OPC sample is determined to be 1047 m2 g−1, which is much higher than that of carbon black, as summarized in Table 2, suggesting that OPC can provide a better support effect than carbon black.TEM images of the Pt/C, PtSn/C, and PtSn/OPC before and after ADT and their particle size distributions are shown in Fig. 1. Pt and PtSn nanoparticles (NPs) are dispersed uniformly on the carbon black and OPC. The average particle size of Pt/C, PtSn/C and PtSn/OPC is found to be 3.0 ± 0.7, 3.0 ± 0.5 and 2.7 ± 0.5 nm. After 1500 cycles of ADT, the mean size increases to about 4.6 ± 1.2, 8.4 ± 1.6 and 4.0 ± 0.8 nm, respectively, because of carbon support corrosion and aggregation, dissolution and migration of the metals, as listed in Table 1. Although the grain growth of Pt and PtSn nanoparticles takes place inevitably during the ADT, the increase in particle size can be suppressed in the PtSn/OPC catalysts, probably owing to the synergistic alloying and support effect.11
| Sample | Particle size (nm) | Surface Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PtO PtO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnOx composition SnOx composition |
HTs | MA (A mgPt−1) | Decay | ECSA (m2 gPt−1) | ||
|---|---|---|---|---|---|---|---|---|
| Fresh | 1500 | Fresh | 1500 | |||||
| Pt/C | 3.0 ± 0.7 | 4.6 ± 1.2 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.321 | 0.028 | 0.004 | 86% | 69.8 |
| PtSn/C | 3.0 ± 0.5 | 8.4 ± 1.6 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
0.310 | 0.063 | 0.014 | 78% | 75.8 |
| PtSn/OPC | 2.7 ± 0.5 | 4.0 ± 0.8 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
0.307 | 0.145 | 0.041 | 72% | 74.1 |
| Sample | Diameter (nm) | SBET (m2 g−1) | Smeso/macro (m2 g−1) | Smicro (m2 g−1) | Vmicro (m2 g−1) |
|---|---|---|---|---|---|
| a SBET – specific surface area, Smeso/macro – mesopore and macropore area, Smicro – micropore area, Vmicro – micropore volume. | |||||
| Pt/C | — | 238 | 168 | 70 | 0.031 |
| PtSn/C | — | 238 | 168 | 70 | 0.031 |
| PtSn/OPC | 180 | 1047 | 504 | 543 | 0.2498 |
Fig. 2 shows the XRD results of catalysts. The diffraction peak at about 25° is from carbon support, and peaks at 39.6, 46.1 and 67.4° is from Pt diffraction of (111), (200), and (220), respectively. The peaks at 34.2 and 45.6° of PtSn/C and PtSn/OPC are associated with Sn oxides, in which the intensity of the oxide peak for PtSn/C is more significant than that for PtSn/OPC, suggesting that different supports may influence not only the interaction but also the phases of the NPs. The diffraction peaks of PtSn/C and PtSn/OPC are slightly shifted to lower angles when compared with those of Pt/C, indicating the lattice expansion owing to the incorporation of Sn and the formation of PtSn alloy.29,30 Therefore, the d-spacing of PtSn/C and PtSn/OPC is determined to be 0.2287 and 0.2293 nm, as listed in Table S1 in ESI,† respectively.
Fig. S2† displays the XPS spectra of Pt/C, PtSn/C and PtSn/OPC. Based on the spectra, the surface compositions of the PtSn catalysts are listed in Table S1† where the surface Pt/Sn composition of PtSn/C and PtSn/OPC is about 54/46 and 60/40, respectively. The Pt 4f spectral profiles for the catalysts are shown in Fig. 3. For Pt/C, the Pt 4f spectra exhibit two characteristic peaks of 71.7 and 75.1 eV due to 4f7/2 and 4f5/2 states. In the PtSn samples, their Pt peaks noted at about 71.0 and 74.0 eV have smaller binding energies than Pt/C, suggesting the presence of more Pt0 state than Pt/C. The Pt spectra of Pt/C after deconvolution have four peaks at 71.7, 72.9, 75.0 and 77.2 eV in which the binding energy at 71.7 and 75.0 eV are corresponded to Pt0 state, and the binding energy at 72.9 and 77.2 eV are corresponded to Pt oxides phases. The Pt/PtO ratio calculated by integration of the Pt spectra peaks is 87/13, 89/11 and 90/10 for Pt/C, PtSn/C and PtSn/OPC, respectively, as compared in Fig. 3. It seems that alloying with Sn and use of OPC as support can modify the surface chemical states of Pt and prevent Pt from oxidation, which is the main mechanism to enhance the ORR performance.14 It has been reported that the surface Pt/PtO ratios of Pt/C changed due to the formation of nanorods, using the graphene support, and alloying with Pd. The formation of surface PtO is inhibited obviously in the graphene-supported PtPd nanorods, suggesting that the synergistic effect of Pd alloying and the graphene support modifies the surface chemical state of the Pt.31 Moreover, Fig. 4 shows the fitting results of Sn 3d5/2 spectra for PtSn/C and PtSn/OPC. The peaks located at 485.9 and 487.5 eV are corresponded to Sn0 and Sn oxides phases, respectively.31 The Sn0/Sn oxide ratio for PtSn/C and PtSn/OPC is 0.3% and 7.0%, respectively, suggesting that most of Sn in the catalysts are oxidized and due to the OPC support effect, more Sn in PtSn/OPC can remain its metallic phase than in PtSn/C. Moreover, the exact Pt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PtO
PtO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn
Sn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2 ratios of catalysts are compared in Table 1. It shows that the OPC support can modify the chemical states of both Pt and Sn.
SnO2 ratios of catalysts are compared in Table 1. It shows that the OPC support can modify the chemical states of both Pt and Sn.
 | ||
| Fig. 3 Pt 4f transition in XPS spectra fitting results with as-prepared Pt/C, PtSn/C and PtSn/OPC catalysts. | ||
 | ||
| Fig. 4 Sn 3d transition in XPS spectra fitting results with as-prepared PtSn/C and PtSn/OPC catalyst. | ||
Fig. S3† shows the XANES spectrum at the Pt LIII edge of the three catalysts. The absorption peak at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 564 eV is ascribed to the Pt electrons transfer from 2p3/2 to 5d5/2 and the differences of white line indicate the degrees of Pt oxidation state.25 In this study, the HTs value of Pt/C, PtSn/C and PtSn/OPC is 0.321, 0.310 and 0.307, respectively. It is straightforward to understand that alloying of Sn can modify the d-band of Pt so that the HTs can decrease from 0.321 to 0.301. Moreover, the use of OPC as the support can modify the d-band further and the HTs of PtSn/OPC can be noted to be 0.307. In the literatures, it has been reported the catalyst with the lower HTs implies lower unfilled d-states, less Pt oxides formation, leading to the promotion of ORR kinetics9,11 and for the graphene-supported Pt and PtPd nanorods, the HTs is about 0.307 and 0.295, respectively. In the view point of Pt d-band vacancy, the effect of Sn alloying and OPC support is similar to that of nanorods structure and graphene support.11 The XANES results are consistent with the XPS data shown in Fig. 3 in which the synergistic effect of Sn alloying and OPC support can modify the electronic states of Pt and suppress its oxidation.
564 eV is ascribed to the Pt electrons transfer from 2p3/2 to 5d5/2 and the differences of white line indicate the degrees of Pt oxidation state.25 In this study, the HTs value of Pt/C, PtSn/C and PtSn/OPC is 0.321, 0.310 and 0.307, respectively. It is straightforward to understand that alloying of Sn can modify the d-band of Pt so that the HTs can decrease from 0.321 to 0.301. Moreover, the use of OPC as the support can modify the d-band further and the HTs of PtSn/OPC can be noted to be 0.307. In the literatures, it has been reported the catalyst with the lower HTs implies lower unfilled d-states, less Pt oxides formation, leading to the promotion of ORR kinetics9,11 and for the graphene-supported Pt and PtPd nanorods, the HTs is about 0.307 and 0.295, respectively. In the view point of Pt d-band vacancy, the effect of Sn alloying and OPC support is similar to that of nanorods structure and graphene support.11 The XANES results are consistent with the XPS data shown in Fig. 3 in which the synergistic effect of Sn alloying and OPC support can modify the electronic states of Pt and suppress its oxidation.
Fig. 5 shows the ORR activity of Pt/C, PtSn/C, and PtSn/OPC. The PtSn/OPC sample has the highest mass activity (MA, current density normalized to Pt loading) among all samples as listed in Table 1. The kinetic current at 0.85 V and specific activity (SA) are also compared in Table S1.† Moreover, after ADT, due to different degrees of Pt dissolution, aggregation, migration and C corrosion,32 decay in ORR performance is noted. Based on the MA results, the decay of Pt/C, PtSn/C, and PtSn/OPC is 86, 78, and 72%, respectively, implying that PtSn/OPC is more chemically stable in the acid environment than PtSn/C and Pt/C, which is consistent with the TEM results displayed in Fig. 1. Fig. S4† displays the CV curve of Pt/C, PtSn/C and PtSn/OPC catalysts. The CV shows a H2 adsorption/desorption region between 0.06 and 0.40 V. The ECSA calculated by measuring of H2 desorption after the deduction of the double-layer region is 69.8, 75.8 and 74.1 m2 gPt−1 for Pt/C, PtSn/C and PtSn/OPC, respectively. Although the OPC has much larger surface area than carbon black, the resulting ECSA of catalysts are similar, maybe attributed to that most of the pores are in the interior. Moreover, the PtSn catalysts have higher ECSA and larger value for double-layer current than Pt/C, attributed to Sn oxides phases or the interaction between the metal and the support.33
 | ||
| Fig. 5 LSV curves of catalysts in 0.5 M H2SO4 with O2 at 1600 rpm with Pt/C, PtSn/C and PtSn/OPC before and after ADT of 1500 potential cycles. | ||
The calculated n values of Pt/C, PtSn/C and PtSn/OPC are approximately 4.0, 3.4 and 3.4, respectively, suggesting that PtSn/C and PtSn/OPC almost complete the reduction to water and have a low hydrogen peroxide production during oxygen reduction.34
Fig. S6† shows the Tafel plots of the catalysts before and after ADT. From the results, it shows that the slope of Pt/C, PtSn/C, and PtSn/OPC is 17, 16, and 16 mV per decade before ADT and 28, 25, 23 mV per decade after ADT, respectively, suggesting that PtSn/OPC with higher onset potential and lower Tafel slope can effectively promote the ORR reaction.
Chronoamperometry tests for the ORR at 0.6 V (vs. Ag/AgCl) were carried out for 1 h and the results are shown in Fig. S7.† The ORR current density of PtSn/C and PtSn/OPC is higher than that of Pt/C. Moreover, the current density of PtSn/OPC is about 2 times higher than that of Pt/C for 1 h, suggesting that the PtSn/OPC catalysts have better stability in acid media.
MA of the catalysts before and after ADT and their HTs are summarized in Fig. 6. It seems that lower HTs implies less Pt oxide formation and higher ORR activity. Moreover, during the ADT, the stabilization stems from electronic modification effect of synergistic Sn and OPC addition.
MA of the current PtSn/C and PtSn/OPC are compared in Table S2† with the MA values reported in the literature.35,36 It shows that PtSn/OPC from this study exhibits a superior ORR performance to other samples reported in the literature, suggesting that the synergic modification of OPC and Sn on Pt is an effective method to promote its ORR activity and stability.
4. Conclusions
In this study, the Pt/C is synergistically modified by Sn alloying and OPC support to enhance the ORR performance. High surface area and ordered porous structure of the OPC support lead to a smaller particle size and narrower size distribution of PtSn particles. The PtSn/OPC has higher activity and long-term durability toward ORR than PtSn/C and Pt/C catalysts. The enhanced ORR performance of PtSn/OPC may stem from the modification of surface chemical states of Pt and Sn as well as the d-band vacancy of Pt.Acknowledgements
The financial support of this work by the National Science Council of Taiwan under grants NSC 102-2622-E-008-010-CC3, NSC 102-2221-E-058-MY2 and NSC 102-2923-E- 008-002-MY3 is greatly appreciated.Notes and references
- B. Lim, M. Jiang, P. H. C. Camargo, E. C. Cho, J. Tao, X. Lu, Y. Zhu and Y. Xia, Science, 2009, 324, 1302 CrossRef CAS PubMed.
- V. R. Stamenkovic, B. Fowler, B. S. Mun, G. Wang, P. N. Ross, C. A. Lucas and N. M. Markovic, Science, 2007, 315, 493 CrossRef CAS PubMed.
- C. W. Liu, Y. C. Wei and K. W. Wang, Chem. Commun., 2010, 46, 2483 RSC.
- C. J. Tseng, S. T. Lo, S. C. Lo and P. P. Chu, Mater. Chem. Phys., 2006, 100, 385 CrossRef CAS.
- B. J. Su, K. W. Wang, T. C. Cheng and C. J. Tseng, Mater. Chem. Phys., 2012, 135, 395 CrossRef CAS.
- Y. J. Hsueh, C. C. Yu, K. R. Lee, C. J. Tseng, B. J. Su, S. K. Wu and L. C. Weng, Int. J. Hydrogen Energy, 2013, 38, 10998 CrossRef CAS.
- C. W. Liu, Y. C. Wei, C. C. Liu and K. W. Wang, J. Mater. Chem., 2012, 22, 4641 RSC.
- C. W. Liu, Y. C. Wei and K. W. Wang, J. Phys. Chem. C, 2011, 115, 8702 CAS.
- T. H. Yeh, C. W. Liu, H. S. Chen and K. W. Wang, Electrochem. Commun., 2013, 31, 125 CrossRef CAS.
- Y. C. Tseng, H. S. Chen, C. W. Liu, T. H. Yeh and K. W. Wang, J. Mater. Chem. A, 2014, 2, 4270 CAS.
- H. S. Chen, Y. T. Liang, T. Y. Chen, Y. C. Tseng, C. W. Liu, S. R. Chung, C. T. Hsieh, C. E. Lee and K. W. Wang, Chem. Commun., 2014, 50, 11165 RSC.
- V. R. Stamenkovic, B. S. Mun, M. Arenz, K. J. J. Mayrhofer, C. A. Lucas, G. Wang, P. N. Ross and N. M. Markovici, Nat. Mater., 2007, 6, 241 CrossRef CAS PubMed.
- J. Zhang, K. Sasaki, E. Sutter and R. R. Adzic, Science, 2007, 315, 220 CrossRef CAS PubMed.
- Y. T. Liang, S. P. Lin, C. W. Liu, S. R. Chung, T. Y. Chen, J. H. Wang and K. W. Wang, Chem. Commun., 2015, 51, 6605 RSC.
- J. S. Yu, S. Kang, S. B. Yoon and G. Chai, J. Am. Chem. Soc., 2002, 124, 9382 CrossRef CAS PubMed.
- E. P. Ambrosio, C. Francia, M. Manzoli, N. Penazzi and P. Spinelli, Int. J. Hydrogen Energy, 2008, 33, 3142 CrossRef CAS.
- S. Q. Song, Y. R. Liang, Z. H. Li, Y. Wang, R. W. Fu, D. C. Wu and P. Tsiakaras, Appl. Catal., B, 2010, 98, 132 CrossRef CAS.
- L. Calvillo, M. Gangeri, S. Perathoner, G. Centi, R. Moliner and M. Lazaro, Int. J. Hydrogen Energy, 2011, 36, 9805 CrossRef CAS.
- J. Song, G. Li and J. Qiao, Electrochim. Acta, 2015, 177, 174 CrossRef CAS.
- J. Tang, J. Liu, N. L. Torad, T. Kimura and Y. Yamauchi, Nano Today, 2014, 9, 305 CrossRef CAS.
- B. J. Su, K. W. Wang, C. J. Tseng, C. H. Wang and Y. J. Hsueh, Int. J. Electrochem. Sci., 2012, 7, 5246 CAS.
- Y. W. Chang, C. W. Liu, Y. C. Wei and K. W. Wang, Electrochem. Commun., 2009, 11, 2161 CrossRef CAS.
- X. S. Zhao, F. Su, Q. Yan, W. Guo, X. Y. Bao, L. Lv and Z. Zhou, J. Mater. Chem., 2006, 16, 637 RSC.
- T. Yangishita, K. Nishio and H. Masuda, Adv. Mater., 2005, 17, 2241 CrossRef.
- S. Mukerjee, S. Srinivasan, M. P. Soriaga and J. McBreen, J. Electrochem. Soc., 1995, 142, 1409 CrossRef CAS.
- D. Chu and S. Gilman, J. Electrochem. Soc., 1994, 141, 1770 CrossRef CAS.
- S. Hwang, G. G. Park, S. D. Yim, S. H. Park, T. H. Yang, H. Kim and C. S. Kim, Fuel Cells, 2010, 10, 245 CrossRef CAS.
- R. Saliger, U. Fischer, C. Herta and J. Fricke, J. Non-Cryst. Solids, 1998, 225, 81 CrossRef CAS.
- J. Asgardi, J. C. Calderón, F. Alcaide, A. Querejeta, L. Calvillo, M. J. Lázaro, G. García and E. Pastor, Appl. Catal., B, 2015, 168–169, 33 CrossRef CAS.
- D. H. Kwak, Y. W. Lee, S. B. Han, E. T. Hwang, H. C. Park, M. C. Kim and K. W. Park, J. Power Sources, 2015, 275, 557 CrossRef CAS.
- F. E. Lopez-Suarez, A. Bueno-Lopez, K. I. B. Eguiluz and G. R. Salazar-Banda, J. Power Sources, 2014, 268, 225 CrossRef CAS.
- A. Pozio, M. D. Francesco, A. Cemmi, F. Cardellini and L. Giorgi, J. Power Sources, 2002, 105, 13 CrossRef CAS.
- S. Beyhan, N. E. Şahin, S. Pronier, J.-M. Léger and F. Kadırgan, Electrochim. Acta, 2015, 151, 565 CrossRef CAS.
- M. Bron, S. Fiechte, M. Hilgendorff and P. Bogdanoff, J. Appl. Electrochem., 2002, 32, 211 CrossRef CAS.
- S. Beyhan, N. E. Şahin, S. Pronier and J. M. Léger, Electrochim. Acta, 2015, 151, 565 CrossRef CAS.
- S. Knani, L. Chirchi, S. Baranton, T. Napporn, J. M. Léger and A. Ghorbel, Int. J. Hydrogen Energy, 2014, 39, 9070 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27439g |
| This journal is © The Royal Society of Chemistry 2016 |