Cellulose nanofibre aerogel filter with tuneable pore structure for oil/water separation and recovery†
Abstract
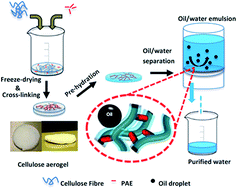
A cellulose nanofibre aerogel filter with tuneable pore structure exhibiting super-hydrophilic and underwater-super-oleophobic behaviours is synthesized by a facile method of cross-linking between cellulose nanofibres and polyamideamine-epichlorohydrin (PAE). The prepared aerogel filter showed excellent oil/water separation efficiency for both oil/water mixtures (100%, even after 10 cycles), and oil/water surfactant-free emulsion (98.6%), which is driven solely by gravity. In addition, the specific structure can de-emulsify oil/water emulsion. The results showed that the cellulose aerogel filter can be used for oil/water separation and recovery.

 Please wait while we load your content...
Please wait while we load your content...