European Viscum album: a potent phytotherapeutic agent with multifarious phytochemicals, pharmacological properties and clinical evidence
Brahma N. Singh
a,
Chaitrali Saha
bc,
Danijel Galun
de,
Dalip K. Upreti
f,
Jagadeesh Bayry
*bcgh and
Srini V. Kaveri
*bcgh
aPharmacognosy & Ethnopharmacology Division, CSIR-National Botanical Research Institute, Lucknow-226 001, India
bInstitut National de la Santé et de la Recherche Médicale Unité 1138, Paris, F-75006, France. E-mail: srini.kaveri@crc.jussieu.fr; jagadeesh.bayry@crc.jussieu.fr; Fax: +33 1 44 27 81 94; Tel: +33 1 44 27 82 01
cCentre de Recherche des Cordeliers, Equipe – Immunopathologie et immuno-intervention thérapeutique, Paris, F-75006, France
dClinic for Digestive Surgery, Clinical Centre of Serbia, Belgrade, 11000, Serbia
eMedical School, University of Belgrade, Belgrade, 11000, Serbia
fLichenology Division, CSIR-National Botanical Research Institute, Lucknow-226 001, India
gSorbonne Universités, UPMC Univ Paris 06, UMR S 1138, Paris, F-75006, France
hUniversité Paris Descartes, Sorbonne Paris Cité, UMR S 1138, Paris, F-75006, France
First published on 22nd February 2016
Abstract
Viscum album L. or European mistletoe (Loranthaceae), a semi-parasitic shrub, has been used as a traditional medicine in Europe for centuries to treat various diseases like cancer, cardiovascular disorder, epilepsy, infertility, hypertension and arthritis. V. album contains diverse phytochemicals, which exert a large number of biological and pharmacological activities. The aim of this review is to compile the developments in the domain of V. album and research trends, with a focus on ethnopharmacology, phytochemistry and pharmacological properties, to illustrate the potential of this phytotherapeutic as an attractive commercial herbal medicine. Crude extracts and isolated chemical constituents from V. album have exhibited significant medicinal effects in experimental models and in patients with cancer, autoimmune and inflammatory conditions. Importantly, recent randomized clinical trials have suggested improved overall survival and quality of life in cancer patients treated with different mistletoe preparations. The current phytochemical studies have shown that lectins, hetero-dimeric glycoproteins, polysaccharides, viscotoxins, alkaloids, lipids, triterpenes, peptides, vesicles, flavonoids, cyclitols and amines are principal bioactive phytochemicals of V. album. Clinical studies and experimental models have revealed that V. album exhibits several pharmacological functions such as immunomodulatory, anti-hypertensive, anti-oxidant, cytotoxicity, anti-tumor, anti-inflammation, anti-diabetic, anti-microbial and sedative activities. It is conceivable that the heterogenous profile of biochemical compounds provides the basis for the broad diversity of pharmacological activities of mistletoe, as each single component contributes diverse modes of action in addition to imparting a synergistic beneficial action in conjunction with other molecules.
1. Introduction
Viscum album L., (Loranthaceae) commonly known as mistletoe or European mistletoe, is recognised by various names: European white-berry mistletoe, bird lime, birdlime, all-heal, and masslin. In German, V. album is called by Mistel, Vogelmistel, Leimmistel, Affolter, and Bocksfutter; gui, gui commun, and gui de druides in French; vischio, visco, vescovaggine, guatrice, pania, and scoaggine in Italian; muerdago in Spanish, and common mistletoe in Asia and Africa.1–4 It is native to Europe and western and southern Asia.5,6 V. album has been commonly used in local medicine in Europe and Asia for thousands of years.2 In Europe, folk medicinal uses of V. album are recorded for curing various ailments such as cancer, hypertension, anxiety, insomnia, headache, internal bleeding and atherosclerosis. The compounds isolated from V. album so far mainly include hetero-dimeric glycoproteins, polysaccharides, lectins, amines, triterpenes, viscotoxins, alkaloids, lipids, peptides, cyclitols, vesicles and flavonoids.7–10 European V. album with its bioactive phytochemicals is possessed with wide-reaching biological activities including immunomodulatory, anti-oxidant, cytotoxicity, anti-tumor, anti-hypertensive, sedative, anti-diabetic and hepato-protective. Meanwhile, V. album has also displayed significant inhibitory bio-activity against human cancer cell lines.11,12 In addition, extract and phytochemicals have ability to inhibit inflammation and to prevent the development of cancer.4,6 A number of phyto-pharmacological providers like WELEDA, ABNOBA HEILMITTEL, HELIXOR HEILMITTEL, NOVIPHARM and MADAUS market a range of different mistletoe preparations. Brand names of various preparations of mistletoe extract are Iscador®, ABNOBAViscum, Cephalektin, Eurixor®, Helixor®, Isorel and Lektinol™.Owing to its extensive use as a potent phytotherapeutic agent in European countries, V. album has been in the spotlight of researchers for a long time. The present review provides an update on the developments in the domain of V. album and research trends, with a focus on ethnopharmacology, phytochemistry, pharmacological properties and clinical use.
2. Botany and distribution
V. album L. is a dioecious, insect-pollinated and hemi-parasitic evergreen shrub that grows on a number of host trees. It is commonly found on the crowns of broad-leaved deciduous trees including apple, ash, hawthorn, lime, cedar of lebanon, larch and other trees. On the oak and pear, it grows very rarely. V. album receives additional nutrients via haustorial attachment to a host, but is also able to photosynthesize. It has 30–100 cm long yellowish and smooth stems with dichotomous branching. The leaves are tongue-shaped, in apposite pairs, broader towards the end, 2–8 cm long, 1–2.5 cm wide, leather textured and of a dull yellow-green colour. Small flowers are inconspicuous and yellowish-green in colour, cluster in the forks of the branches and 2–3 cm diameter. Neither male nor female flowers have a corolla. The fruit is a white or yellow berry, smooth, ripening in December, containing one (very seldom) seed covered in the very sticky pulp.13 Based on the fruit colour, leaf shape and size, and most obviously the host trees, numerous subspecies of V. album have been classified. These include V. album subsp. abietis (Wiesb.) having white fruit and leaves up to 8 cm; V. album subsp. album having white fruit and leaves 3–5 cm; V. album subsp. austriacum (Wiesb.) having yellow fruit and leaves 2–4 cm; V. album subsp. meridianum (Danser) having yellow fruit and leaves 3–5 cm; V. album subsp. creticum having white fruit and short leaves; and Viscum album subsp. coloratum Kom, which is now considered as a separate species Viscum coloratum (Kom) Nakai by the Flora of China.Geographically, Mistletoe is distributed from North Africa to Southern England and Southern Scandinavian regions, across Central Europe to Southwest and East Asia to Japan. In Europe, three subspecies have been identified depending on the species of host trees. V. album subsp. album grows on hardwoods, V. album subsp. abietis uses fir as host tree and V. album subsp. laxum is dependent on pines and spruce. V. album with coloured fruits are also recorded in further east.13 In United Kingdom, V. album is distributed from East Devon to Yorkshire, and is exceptionally common across London and regions of Central and Southern England.
3. Traditional uses and ethnopharmacology
The European V. album is a pharmaceutical plant and a symbol in mythology. It is the first plant, which was termed as “mistletoe”. According to G. P. Secundus (23–79 AC) this plant was considered to be an antidote for poisons and the plant became a miracle because of its ability to cure each illness.Traditionally, mistletoe infusion has been used for high blood pressure, dizziness and hives. Ancient Greek records suggest that during 460–377 BC, spleen-related diseases were treated using Oak tree mistletoe. During 23–79 AC, Plinius explained the beneficial role of mistletoe in the treatment of infertility, ulcers and epilepsy. Platonist around 150 AC, described the utilization of mistletoe to treat tumors. In a French work on domestic remedies, in the year 1682, it was considered as a golden herb for treating epilepsy. During the year 1731, mistletoe was used for various purposes including labour pain and deworming in children. Later, it had been used for curing convulsions delirium, hysteria, neuralgia, nervous debility, urinary disorders, heart disease and many other complaints arising from a weakened and disordered state of the nervous system. Mistletoe extracts contain several toxic components, several of which are lectins, or proteins capable of binding to specific sugars. In 1921, the Austrian anthroposophical spiritual leader Rudolf Steiner recommended that mistletoe could be used to treat cancer, based on the observation that mistletoe, like cancer, is a parasitic and lethal to its host. Swiss and German clinics were founded to implement this idea and still actively use mistletoe preparation fermented with a strain of Lactobacillus for 3 days.
Despite having a strong historical background of mistletoe, in the 19th century scientific community rejected mistletoe remedy and the interest was re-awakened in the 20th century when Gaultier demonstrated oral/subcutaneous administration of fresh mistletoe extract to cure blood pressure-related issues both in animals and humans. Traditionally, the European mistletoe has been widely used for many years with remarkable therapeutic effects for the treatment of hypertension, anxiety, insomnia, internal bleeding, atherosclerosis and in complementary cancer therapies. In the year 1920, the founder of anthroposophy, Rudolf Steiner, introduced V. album as an anti-cancer remedy.14 Although local medicine at the end of the 19th century still regarded mistletoe as a crucial part of the medicine box, academic medicine in the growing scientific age lost considerable attention in mistletoe as a remedy.
4. Bioactive constituents of European V. album
European mistletoe is characterized by a number of phytochemicals including lectins, polysaccharides, alkaloids, terpenoids, proteins, amines, peptides, polyphenols, flavonoids, phytosterols and amino acids (Table 1). Interestingly, some therapeutic phytochemicals such as certain alkaloids are not produced by the mistletoe rather absorbed from the host tree.| S. no. | Chemical constituents | Content | Source | References |
|---|---|---|---|---|
| 1 | Viscotoxins | 0.05–0.1% | 18 | |
| Isoforms: A1, A2, A3, B, B2, C1 & 1-PS | Leaves | 17 | ||
| Stem | ||||
| 2 | Lectins | 0.34–1.0 mg g−1 dried material | Older stems | 22 and 24–26 |
| Isoforms: ML-I, ML-II, & ML-III | ||||
| 3 | Carbohydrates | 44–58%/dry wt | Leaves | 28 |
| Stem | ||||
| Berries | ||||
| Methylated homogalacturonan | Stem | 30 | ||
| Pectin | Berries | 18 | ||
| Leaves | ||||
| 1→α4-Galacturonic acid methyl ester | Berries | 29 | ||
| Arabinogalactan | Berries | 28 | ||
| 4 | Polyphenols and phenylpropanoids | 50–85 mg/100 g dry wt | Leaves | 33 |
| Stem | ||||
| Berries | ||||
| Flavonoids | 35 and 39 | |||
| Phenolic acids | 33 | |||
| Lignans | 40 | |||
| Kalopanaxin D | 43 | |||
| 5 | Vitamin C | 750 mg/100 g fresh wt | Leaves | 18 |
| Berries | ||||
| 6 | Proteins | 9.3% | Leaves | 136 |
| Stem | ||||
| Berries | ||||
| 7 | Lipophilic compounds | — | ||
| Terpenoids | Leaves | 47 | ||
| Stem | ||||
| Berries | ||||
| Phytosterols | Berries | 46 | ||
| Saturated fatty acids | Leaves | 48–50 | ||
| Stem | ||||
| 8 | Inorganic elements | |||
| Potassium, calcium, manganese, sodium, nickel, phosphate, selenium, silica, magnesium, and zinc | — | Leaves | 18 | |
| Stem | ||||
| 9 | Others | |||
| Cyclic peptides, alkaloids, amines (histamine and cetylcholine), jasmonic acid, cysteine, glutathione, and xanthophyll | — | Leaves | 54 | |
| Stem | ||||
| Berries |
4.1. Viscotoxins
Viscotoxins, a mixture of low-molecular weight cysteine-rich and basic proteins belong to plant thionins (α and β) and are synthesized in the leaves and stems.15,16 They are amphipathic in nature consisting of 46 amino acids with a molecular mass of 5 kDa. The polypeptide chains are attached through three or four disulphide bonds at highly conserved positions (Cys3/Cys40, Cys3/Cys32, and Cys16/Cys26), giving them a compact structure and high stability against denaturing conditions such as heat and proteases. To date, seven different isoforms have been characterized (A1, A2, A3, B, B2, C1 and 1 PS) and are differed mainly in their sequence of amino acids.17 The content of viscotoxins varies from 0.05 to 0.1%, while composition depends on the host tree. For example, the presence of viscotoxins A2 and A3 were observed in V. album ssp. album (VAA), however the predominance of PS-V was detected in V. album ssp. austriacum. All viscotoxins, with the exception of A2, were detected but A3 was predominant in V. album ssp. abietis.18Investigation on the 3D-structures of viscotoxins also provided information on a specific phosphate-binding site.19 It has also been assumed that the phosphate-binding site and amphipathic structures of the viscotoxins promote cytotoxicity in eukaryotic cells by interfering with cell membrane and altering its integrity. In addition to their high structural homology, biological effect of viscotoxins can vary according to their isoforms.20 The viscotoxins reveal a high structural and pharmacological association with snake (cobra) cardiotoxins.21
4.2. Mistletoe lectins (MLs)
The main compounds isolated from V. album are MLs (a mixture of high-molecular-weight polypeptides) and the total content is in the range of 340–1000 μg g−1 dried plant material or their content is not less than 2% of total polypeptides and proteins.22,23 The lectin content is highest in the winter. Sprouts and shoots contain the highest concentrations. Three different MLs (ML-I, ML-II, and ML-III) with differential sugar-binding specificities have been isolated from European mistletoe by affinity chromatography on partially hydrolyzed Sepharose and human immunoglobulin-Sepharose.18,24,25 These include galactose-specific ML-I (115 kDa, dimer), galactose- and N-acetyl-D-galactosamine-specific ML-II (60 kDa) and N-acetyl-D-galactosamine-specific ML-III (60 kDa). All three MLs have high reactivity with human erythrocytes without specificity for the A, B, and O blood groups.Peumans and colleagues reported that deciduous trees contain mostly ML-I and European mistletoe growing on fir and pine trees found to be rich in ML-III.22 The subdomains of ML-I and ML-III were identified to be responsible for sugar binding. These authors also first described chitin-binding (cb) MLs with a molecular weight of 10.8 kDa. The primary structures of three cbMLs including cbML-1, cbML-2, and cbML-3 are very closely related to hevein. MLs are categorized as type-2 ribosome-inactivating proteins, which consist of two peptide chains namely chain A comprising three distinct individual domains and chain B having two domains with similar configurations.18,26,27 The chains are linked by a disulfide bridge. The chain A inhibits protein synthesis by degrading the 28S rRNA in ribosomes of eukaryotic cells and also accelerates apoptosis. While, the chain B is capable of binding to glycoconjugates of cell surface and thereby permitting the cellular entry of toxic subunit.22 Based on glycosylation patterns of MLs, more than 20 different isoforms have been separated by isoelectric focusing.
4.3. Carbohydrates
Further constituents of European V. album include oligo- and polysaccharides. Structurally different types of mistletoe polysaccharides have been identified in the berries and leaves. A highly methylated homogalacturonan, pectin (42 kDa), 1→α4 galacturonic acid methyl ester and arabinogalactan (110 kDa) were characterized in leaves and stem, while berries were specially rich in other polysaccharides such as rhamnogalacturonans with arabinogalactan side chains (1340 kDa), arabinogalactans and small amounts of xyloglucans.28–30 Monosaccharides and polyols were also identified, but after hydrolysis of mistletoe extract. The content of polysaccharides is varied depending on the host plants. For example, inositol (58%) was recorded at early stage of lime tree, however in the latter stage, galactose (44%) was dominant.4.4. Polyphenols and phenylpropanoids
A range of flavonoids, phenylpropanoids, and phenolic acids were isolated from European V. album and the host tree has an influence on their contents. For example, high contents of salicylic acid and rosmarinic acid were detected in Sorbus aucuparia and Malus domestica, respectively. It has also been noticed that mistletoe grown on Fraxinus excelsior had the highest quantity of total phenolic acids and total flavonoids. The diversified qualitative and quantitative amount of phenolic acids including caffeic, phydroxybenzoic, salicylic, protocatechuic, ferulic, and sinapic acids were observed in a free state and as glycosides.31,32 Recently, two new phenolic acids have been isolated from European white-berry V. album, including gallic acid and 3-(3′-carbomethoxypropyl)-7→3′′-protocatechoyl galloate.33Two classes of flavonoids such as chalcones and flavanones were isolated from alcoholic extract of V. album in their glycosidic form and with methoxyl groups in the molecules.18 They were 5,7-dimethoxyflavanone-4′-O-[2′′-O-(5′′′-O-trans-cinnamoyl)-apiosyl]-glucoside, 2′-hydroxy-4′,6′-dimethoxychalcone-4-O-[2′′-O-(5′′′-O-trans-cinnamoyl)-apiosyl]-glucoside, 5,7-dimethoxy-flavanone-4′-O-glucoside, 2′-hydroxy-4′,6′-dimethoxychalcone-4-O-glucoside, 2′-hydroxy-3,4′,6′-trimethoxychalcone-4-O-glucoside, 5,7-dimethoxyflavanone-4′-O-[apiosyl-(1→2)]-glucoside and (2S)-3′,5,7-trimethoxyflavanone-4′-O-glucoside, and 2′-hydroxy-4′,6′-dimethoxychalcone-4-O-[apiosyl(1→2)] glucoside.34,35 A promising anti-oxidant flavonoid quercetin has been detected only after acid hydrolysis of V. album extract.36 Other flavonoids including quercetin, kaempferol and their mono-, di- and trimethylethers were also characterized in epicuticular waxes of different subspecies of the European mistletoe.37 Chaudhary and colleagues reported that 80% methanolic extract of mistletoe contains many polyphenolic constituents viz. 5,7-dimethoxy-4′-hydroxy flavanone, 3-(4-acetoxy-3,5-dimethoxy)-phenyl-2E-propenyl-β-glucoside, 5,7-dimethoxyflavanone-4′-O-β-glucoside, 4′-O-[bapiosyl(1→2)]-β-glucosyl]-5-hydroxy-7-O-sinapylflavanone, 3-(4-hydroxy-3,5-dimethoxy)-phenyl-2E-propenyl-β-glucoside and 4′,5-dimethoxy-7-hydroxy flavanone.38
Recently, four new flavonoid glycosides were isolated and identified from the leaves and twigs of V. album such as 3,7,3′-tri-O-methylquercetin-4′-O-β-D-apiofuranosyl-(1→2)-O-β-D-glucopyranoside, 7,3′-di-O-methylquercetin-4′-O-β-D-glucopyranosyl-3-O-[6′′′-(3-hydroxy-3-methylglutaroyl)]-α-D-glucopyranoside, 7,3′-di-O-methylquercetin-4′-O-β-D-glucopyranosyl-3-O-[(6′′′′→5′′′′)-O-1′′′′′-(sinap-4-yl)-β-D-glucopyranosyl-6-(3-hydroxy-3-methylglutaroyl)]-α-D-glucopyranoside and (2S)-5-hydroxy-7,3′-dimethoxyflavanone-4′-O-β-D-apiofuranosyl-(1→5)-O-β-D-apiofuranosyl-(1→2)-O-β-D-glucopyranoside.39
Phenylpropanoids are the important bioactive molecules of the European mistletoe, with extremely diverse structures and wide-spectrum medicinal effects. The leaves and stem were found to contain several phenylpropanoids including coniferyl alcohol 4-O-β-D-glucoside (coniferin), syringenin 4-O-β-D-glucoside (syringin), syringenin 4-O-β-D-apiosyl(1→2)-β-D-glucoside, and lignans such as syringaresinol 4′,4′′-di-O-glucoside (eleutheroside E) and syringaresinol-O-glucoside.40,41 Lignan skeleton also contained trihydroxy-tetramethoxy-epoxy glucosides such as ligalbumosides A to E and alangilignoside C.42 The quantity of phenylpropanoids also varied according to the mistletoe subspecies. Maximum levels of syringin and coniferin were noticed in the extract of VAA by HPLC, having isocratic mobile phase (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 N sodium acetate; 20
0.1 N sodium acetate; 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73.5
73.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5). Apart from these phenylpropanoids, kalopanaxin D (4-[2-O-(apiosyl)-β-D-glucosyloxy]-3-methoxycinnamyl alcohol) was detected. V. album ssp. austriacum contains trace amount of coniferin, and both syringin and coniferin were characterized in V. album ssp. abietis. However, both these subspecies of mistletoe were unable to synthesize kalopanaxin D.43
6.5). Apart from these phenylpropanoids, kalopanaxin D (4-[2-O-(apiosyl)-β-D-glucosyloxy]-3-methoxycinnamyl alcohol) was detected. V. album ssp. austriacum contains trace amount of coniferin, and both syringin and coniferin were characterized in V. album ssp. abietis. However, both these subspecies of mistletoe were unable to synthesize kalopanaxin D.43
4.5. Lipid soluble compounds
Terpenoids, liposoluble compounds are the main components of European V. album. The lipid soluble extract of V. album showed the presence of oleanolic acid, β-amyrin acetate, β-amyrinacetate, lupeol, lupeol acetate, betulinic acid and ursolic acid.44,45 A mixture of phytosterols including β-sitosterol and stigmasterol, and their esters were also identified.46,47 Moreover, other lipophilic compounds specially saturated palmitic, arachidic, lignoceric, behenic and cerotic acids and the unsaturated oleic, linoleic and linolenic acids have been identified in the Turkey V. album extracts.48,49 Long-chain fatty acids and hydrocarbons including loliolide, vomifoliol trans-α-bergamotene and trans-β-farnesene have been detected in the extracts obtained from supercritical fluid extraction method.50 Due to poor solubility of triterpene in water, it is difficult to extract from the mistletoe. However, 2-hydroxypropyl-β-cyclodextrin and sodium phosphate (pH 7.3) as solubilizers have been developed and represent excellent tools for the extraction of triterpenes.47,514.6. Trace minerals
In addition to organic components, mistletoe contains trace mineral elements including potassium, calcium, manganese, sodium, nickel, phosphate, selenium, silica, magnesium and zinc. Importantly, calcium has been detected mainly in its non-soluble oxalate form.52,53 Mistletoe grown on oak and fir have high levels of manganese.534.7. Other chemical constituents
Cyclic peptides, amino acids, proteins (9.3%), alkaloids, amines (histamine and cetylcholine), jasmonic acid, cysteine, glutathione, vitamin C and xanthophyll are the other constituents of V. album.54,55 Three new diarylheptanoids including (3S,5R)-3-hydroxy-5-methoxy-1,7-bis(4-hydroxyphenyl)-6E-heptene (1), (3S,5S)-3-hydroxy-5-methoxy-1,7-bis(4-hydroxyphenyl)-6E-heptene (2) and (3S)-3-hydroxy-1,7-bis(4-hydroxyphenyl)-6E-hepten-5-one have been isolated and determined from the leaves and twigs of V. album.39 Arabinogalactan proteins (APs) were isolated from berries and aerial part of European mistletoe. The ratios of arabinose and galactose in APs are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.7 and 1
0.7 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.18 in barriers and aerial part, respectively.
1.18 in barriers and aerial part, respectively.
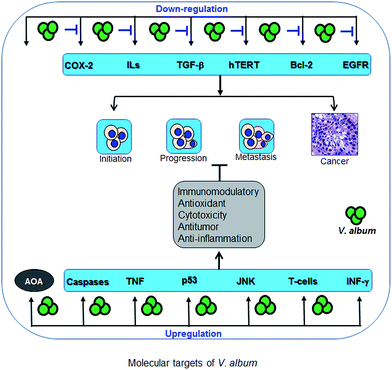
5. Multifarious pharmacological properties
V. album has been used as a folk herbal remedy in Europe and was a mythical shrub that had a strong influence on people in ancient times. Owing to the presence of a range of therapeutic and bioactive chemical constituents, European mistletoe exhibits multifarious pharmacological activities by altering molecular events in the cells (Fig. 1 and Table 2).| Bioactivity | Extract/constituent | Model system | Mechanism | Dose | References |
|---|---|---|---|---|---|
| Antioxidant activity | Extracts of V. album grown on lime tree or white locust tree | HeLa cells | Inhibits mitochondrial DNA damage induced by H2O2 | 10 μg mL−1 for 48 h | 110 |
| Organic extracts | In vitro | Show anti-glycation and antioxidant properties | 38 | ||
| Lectin | LLC-PK(1) cells | Exhibits free radical scavenging, expression of cyclooxygenase-2, inducible NO synthase, SIN-1-induced nuclear factor kappa B and the phosphorylation of inhibitor kappa B alpha | IC50 42.6 μg mL−1 | 111 | |
| Methanolic extract | Rats | Shows DPPH radical scavenging and anti-lipid peroxidation activities | 500 mg kg−1 | 112 | |
| Anti-inflammatory | Preparation (VA Qu Spez) | A549 cells | Inhibits prostaglandin E2, by selectively inhibiting COX-2 | 100 μg mL−1 | 6 |
| Preparation (VA Qu Spez) | A549 cells | Reduces COX-2 mRNA half-life | 50 μg mL−1 | 4 | |
| Flavonoids | Rats | Inhibit carrageenan-induced hind paw edema without any toxic effects | 30 mg kg−1 | 60 | |
| Preparation (Isorel) | Mice | Triggers abundant tumour necrosis with inflammatory response, oedema and destruction of the malignant tissue | 100 mg kg−1 | 61 | |
| Immunomodulatory | Preparation (QU FrF) | B16 mouse melanoma | Abrogates IL-12 expression | 20 μg per mouse per day | 190 |
| VA Qu Spez | Dendritic cells | Stimulates proliferation of CD4+ T cells | 5–15 μg mL−1 | 69 | |
| N-Acetyl-D-galactosamine-specific lectin | T Cells | Inhibits 3000 immune functions-regulating genes | 600 ng mL−1 | 70 | |
| KME | Epinephelus bruneus | Enhances phagocytic activity | 1% and 2% | 71 | |
| Aviscumine | In vivo and clinical phase I studies | Activates immune system | 1.5 mg per kg per day | 72 | |
| KML | Tumoral implantation | Modulates lymphocytes, natural killer cells, and macrophages | 74 | ||
| VCA | Murine splenocytes | Decreases interferon (IFN)-gamma secretion | 4–64 ng mL−1 | 80 | |
| VCA | hPBMC cells T-lymphocytes | Releases IL-1α, IL-1β, IL-6, IL-8, and IFN-γ | 4–16 pg mL−1, 4–32 pg mL−1 | 62 | |
| Lectin | T-Lymphocytes | Enhances expression of IL-1 alpha, IL-1 beta, IL-6, IL-10, TNF-α, interferon-gamma, and granulocyte-monocyte colony stimulating factor genes | 1–8 ng mL−1 | 62 | |
| ML-I | Peripheral blood mononuclear cells | Induces cytokines gene expression and protein production | 1 ng mL−1 | 82 | |
| ML-I | Peripheral blood mononuclear cells | Induces IL-6 and TNF-alpha production | 10 ng mL−1 | 82 | |
| VAA extract | Epithelial cells | Stimulates the levels of CD4(+)CD25(+) and CD8(+)CD25(+) T cells and CD3(−)CD16(+)CD56(+) natural killer cells | 10% ethanolic extract | 83 | |
| VTA1 (85 nm), VTA2 (18 nm) and VTA3 | K562 and NK effector cells | Increase natural killer cell-mediated cytotoxicity | 6–25 nM | 85 | |
| Viscotoxins | Rats | Enhance phagocytosis and burst activity against E. coli infection | 25 and 250 μg mL−1 | 86 | |
| Aqueous extract | Human trail | Induces the secretion of Th1-(IFN-γ) or Th2-(IL-4) | 87 | ||
| rVAA | Rat splenocytes | Enhances the secretion of an active form of IL-12 | 100 pg mL−1 | 89 | |
| Iscador Pini | Peripheral blood mononuclear cells | Activates T-helper cells (CD4+) | 0.1–1.0 mg mL−1 | 88 | |
| Cytotoxicity | Mistletoe lectin | SK-Hep-1 (p53-positive) and Hep 3B (p53-negative)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cells cells |
Induces apoptosis by stimulating mitochondrial membrane potential (MMP) breakdown and stimulating caspase-3 | 10–50 ng mL−1 | 91 |
| VA Qu FrF | CEM, HL-60 and MM-6 cells | Reveals cell cytotoxicity | 100–200 μg mL−1 | 92 | |
| Viscotoxin-free V. album extract | Enhances granulocyte activity | 93 | |||
| Preparations | Daoy, D342, D425 and UW-288-2 cells | Induces cytotoxicity by reducing mitochondrial activity | 50 mg mL−1 | 94 | |
| MLI, MLII, and MLIII | Molt 4 cells | Exhibits cytotoxic activity | 95 | ||
| Viscotoxins and alkaloids | Tumor MSV cells | Show cytotoxicity | 10 μg mL−1 | 97 | |
| MLI | Exhibits cytotoxicity | 96 | |||
| KML-C | Human and murine tumor cells | Shows strong cytotoxicity by inducing apoptotic cell death | 0.4–307 mg mL−1 | 98 | |
| Anti-angiogenic | ME | B16L6 melanoma cells | Suppresses tumor growth and metastasis by elevating fragmentation and nuclear morphological changes | 100 ng mL−1 | 191 |
| VA QU FrF | Human umbilical vein endothelial and immortalized human venous endothelial cells | Induces apoptosis | 12.5–50 μg mL−1 | 12 | |
| FME | Glioblastoma cells | Regulates cytokine TGF-β and matrix-metallo-proteinases central genes expression | 100 μl mL−1 | 100 | |
| VAA-I | PLB-985 and chronic granulomatous disease cells | Induces apoptosis via caspase activation | 1 mg mL−1 | 101 | |
| VAA-I | LPS-treated human neutrophils and murine neutrophils | Activates apoptosis | 1–100 ng mL−1 | 102 | |
| VAA-I | Human neutrophils | Induces apoptosis via acceleration the loss of antiapoptotic Mcl-1 expression and the degradation of cytoskeletal paxillin and vimentin proteins | 1–10 mg mL−1 | 103 | |
| Iscador Qu | Endothelial cell cultures | Causes early cell cycle inhibition followed by apoptosis | 104 | ||
| Epi-oleanolic acid | Human and marine cancer cells | Activates apoptotic cell death, characterized by morphological changes and DNA fragmentation | 4, 20, and 100 μg mL−1 | 105 | |
| VCA | Hepatocarcinoma Hep3B cells | Induces apoptosis by increasing ROS production and a loss of DeltaPsim | 20 ng mL−1 | 106 | |
| β-Galactoside, N-acetyl-D-galactosamine-specific lectin II, polysaccharides, viscotoxin | U937 cells | Induce apoptosis through activation of the phosphotransferase activity of c-Jun N-terminal kinase 1 (JNK1)/stress-activated protein kinase (SAPK) | 100 ng mL−1 | 107 | |
| Viscotoxins | Lymphocytes | Induce cell death by producing mitochondrial Apo2.7 molecules and by generating ROS-intermediates | 100 ng mL−1 | 174 | |
| Anti-tumoral | Aqueous extract | Cancer cells | Depletion of hypoxanthine concentration and xanthine oxidase activation | 113 | |
| Lipophilic extract and its predominant triterpene oleanolic acid | Tumour cells | Decreases MCP-1 induced monocyte transmigration | 25 μg mL−1 | 114 | |
| Ethanolic extract containing viscotoxin | Swiss female mice | Enhances the anti-tumor effect of doxorubicin | 50 mg kg−1 | 115 | |
| VAE | C6 glioma cells | Induces apoptosis by activating caspase-mediated pathway | 100 μg mL−1 | 116 | |
| VAA-1 | Lung carcinoma A549 | Enhances anti-proliferating potential of cycloheximide by inducing G1-phase accumulation | 117 | ||
| Synergistic effect of mistletoe extract and its components | U937 cells | Induce apoptosis via activation of caspase cascades | 100 ng mL−1 | 118 | |
| ML-II | U937 cells | Enhances apoptotic response through augmentation of Fas/Fas L expression and caspase activation | 100 ng mL−1 | 119 | |
| ML-III | Tumour cell lines and human lymphocytes | Reduce the expression of nuclear p53 and Bcl-2 | 120 | ||
| Oleanolic acid | HaCaT cells | Induces apoptosis by altering cellular morphology as well as DNA integrity | 12.5–200 μM | 121 | |
| Either solubilized triterpene acids or lectins and combinations thereof | Acute lymphoblastic leukaemia cell line NALM-6 | Induce dose-dependent apoptosis via caspase-dependent pathways | 8 ng mL−1 | 122 | |
| VE from apple and pine | Human macrophages | Increases anti-tumoral activity of activated human macrophages by inducing the production of NO | 123 | ||
| Lectin containing Iscador M Spezial and Iscador Qu Spezial | Mammary cancer MAXF 401NL cells | 70% growth inhibition | 15 μg mL−1 | 124 and 125 | |
| VAE | Bladder carcinoma T24, TCCSUP, J82 and UM-UC3 cell lines | Induces necrosis and apoptotic cell death | 10–1000 μg mL−1 | 126 | |
| Viscotoxin B2 | Rat osteoblast-like sarcoma 17/2.8 cells | Exhibits antitumor activity | IC50 1.6 mg L−1 | 127 | |
| Viscin | Molt4 U937 leukaemia cells | Inhibits growth and induce apoptotic cell death | IC50 118 ± 24 & 138 ± 24 μg mL−1 | 48 | |
| VAC | Hepatoma cells | Induces apoptosis by decreasing Bcl-2 level and telomerase activity and by inducing of Bax | 10 ng mL−1 | 128 | |
| Lectins | Rat liver | Modulate protein kinase activities | 100 μg mL−1 | 129 | |
| Lectin | Yac-1 tumor cells | Increases natural killer-mediated cytotoxicity | 50 ng per mouse | 130 | |
| KM-110 | B16-BL6, 26-M3.1, L5178Y-ML25 cells | Inhibits lung metastasis | 100 μg per mouse | 131 | |
| Iscador | Melanoma cells in mice | Inhibits lung metastasis by reducing nodule formation (92%) and by enhancing a life span (71%) | IC50 0.0166 mg per dose | 132 | |
| ME | Mice | Inhibits pulmonary metastatic colonization | 3, 30 or 150 ng kg−1 | 133 | |
| KM-110 | L5178Y-ML25 lymphoma cells | Inhibit liver and spleen metastasis | 100 μg mL−1 | 131 | |
| Anti-diabetic | VAC | Mice | Enhances the insulin secretion from the pancreatic β-cell without any effects of cytotoxicity | 2 mg mL−1 | 55 |
| VAC | Mice | Upregulates pattern of insulin genes such as PDX-1 and β2/neuroD | 2 mg mL−1 | 55 | |
| ML-I | Mimics the sugar compound | 137 | |||
| VE | Shows potent alpha-glucosidase inhibitory activity | IC50 10.1 mg mL−1 | 138 | ||
| Aqueous extract | Stimulate secretion of insulin (1.1 to 12.2-fold) from clonal pancreatic B-cells | 1–10 mg mL−1 | 139 | ||
| Anti-hypertensive | Ethanol extract | Wistar rats | Reduces the blood pressure | 3.33 × 10−5 mg kg−1 | 140 |
| Anti-microbial | Methanolic extract | Pathogenic microorganisms | Shows antimicrobial activity | 141 | |
| Aqueous extract | Vero cells | Prevent HPIV-2 replication and the virus production | 1 μg mL−1 | 142 | |
| Anti-mutagenic | VAC | Salmonella typhimurium strains TA98 and TA100 | Prevents the mutagenicity of the indirect-acting mutagen 2-aminoanthracene | 100–400 μg mL−1 | 145 |
| Anticonvulsant | VE | 4.5 year old girl | Manages refractory childhood absence epilepsy | 146 | |
| Wound healing activity | Lipophilic extract | Rats | Stimulates migration of NIH/3T3 fibroblasts | 10 μg mL−1 | 147 |
| Anti-ageing | VAC | Caenorhabditis elegans and Drosophila melanogaster | Promotes the mean survival time | 50 μg mL−1 | 148 |
| Anti-obesity | VAC | Mice | Protects against hepatic steatosis | 3 g per kg per day | 149 |
| Endurance promoting | KME | Cell lines | Activates PGC-1α and SIRT1 | 400 & 1000 mg mL−1 | 150 |
| Others | Aqueous extract of leaves | Mice and rats | Exhibits sedative, antiepileptic and antipsychotic activities | 50 & 150 mg mL−1, p.o. | 2 |
5.1. Anti-inflammatory
Our group has found that VA Qu Spez impedes cytokine-induced prostaglandin E2 (PGE2), by selectively inhibiting cyclooxygenase-2 (COX-2), which is transcriptionally activated in response to various pro-inflammatory cytokines.6 Further dissection of the molecular events revealed that V. album significantly reduces the COX-2 mRNA half-life without influencing its protein stability, implicating that V. album induces destabilization of COX-2 mRNA.4 Excessive amount of PGE2 is also associated with pro-tumoral conditions and enhances dendritic cell-mediated regulatory T cell expansion.56–59 Thus, inhibition of PGE2 by V. album was also important for anti-tumoral functions. To assess the anti-nociceptive and anti-inflammatory activities of isolated flavonoids of V. album, p-benzoquinone-induced writhing test and carrageenan-induced hind paw edema model were used, respectively. The ethyl acetate fraction (250 mg kg−1) as well as 2′-hydroxy-4′,6′-dimethoxy-chalcone-4-O-beta-D-glucopyranoside and 5,7-dimethoxy-flavanone-4′-O-[β-D-apiofuranosyl-(1→2)]-β-D-glucopyranoside at the concentration of 30 mg kg−1 dose were shown to possess remarkable anti-nociceptive and anti-inflammatory activities, without inducing any apparent acute toxicity as well as gastric damage.60A single intraperitoneal (IP) injection of the mistletoe preparation Isorel (100 mg kg−1) decreased the size of the tumour and triggered abundant tumour necrosis with inflammatory response, oedema and destruction of the malignant tissue.61 Moreover, the Isorel-treated melanoma cells were found to be more sensitive to the cytotoxic activity of the lymphocytes in the presence of Isorel-treated mice plasma than the control tumour cells.
5.2. Immunomodulatory
V. album and their chemical constituents have well-known immunomodulatory properties. V. album significantly enhanced interferon gamma (IFN-γ) responses.62 Our group has reported that VA Qu FrF mistletoe preparation significantly inhibits tumor growth in vivo in an IL-12-dependent mechanism.63 As dendritic cells are key players in regulating the immune responses,64–68 we examined the effect of V. album on these innate cells. V. album Qu Spez enhanced the expression of several antigen-presenting and co-stimulatory molecules on human dendritic cells and additionally induced secretion of pro-inflammatory cytokines such as IL-6 and IL-8, and stimulated the proliferation of CD4+ T cells.69 A transcriptome analysis of the gene expression profile in human T cells following incubation with N-acetyl-D-galactosamine-specific lectin of V. album var. coloratum agglutinin (VCA, Korean mistletoe lectin) revealed activation and inhibition of 3000 genes, which are involved in a wide range of immune functions. These genes were related to cytokines, cell adhesion, cell motility, cell growth and maintenance, cell death and the response to stress and external stimulus.70A diet enriched by 1% and 2% of Korean mistletoe extract positively enhanced innate immune responses such as respiratory burst and phagocytic activity in kelp grouper Epinephelus bruneus against Philasterides dicentrarchi.71 A recombinant form of Escherichia coli, producing ML (aviscumine) was developed. Immunomodulatory and cytotoxic activities have been observed in in vivo and clinical phase I studies.72 The natural killer (NK) cells have been anticipated as one of the candidates for direct tumour cell destruction.73 Under in vitro and in vivo systems, Korean mistletoe lectin was found to enhance the immune system through modulation of lymphocytes, NK cells and macrophages.74 Subcutaneous (SC) administration of mistletoe causes increase in relative number of lymphocytes with activated phenotype, NK cells and specific subsets of lymphocytes including B cells, CD4+ T cells and cytotoxic T cells.75 These results were also confirmed in other studies and found that V. album treatment can result in normalization of initial immune indices.25,76–78 Further, mistletoe extracts obtained from apple (mali) or pine (pini) induced in vitro oligoclonal activation of CD4+ T cells from mistletoe-treated cancer patients.77 A placebo-controlled study in healthy individuals found that Iscador Quercus causes eosinophilia due to stimulation of IL-5 and GM-CSF by ML.78 Non-toxic doses of ML-1 or its carbohydrate-binding subunit prompted significant increase in components of the cellular host defense system including NK cell cytotoxicity or release of various cytokines including IL-1, TNF-α and IL-6.79
The effect of VCA on murine splenocytes was investigated to examine whether VCA acts as an immunomodulator. VCA in a dose-dependent manner (4–64 ng mL−1) decreased IFN-γ secretion in concanavalin A (ConA)-stimulated murine splenocytes without changing IL-4 levels.80 Treatment of VCA also resulted in an anti-proliferative effect at 2–8 ng mL−1 and 1–8 ng mL−1 in human peripheral blood mononuclear cells (hPBMC) and T lymphocytes, respectively. However, at lower doses (4–16 pg mL−1 and 4–32 pg mL−1 respectively), a proliferative effect was noticed in hPBMC and T lymphocytes.62 The RT-PCR results confirmed the release of pro-inflammatory cytokines such as IL-1α, IL-1β, IL-6, IL-8 and IFN-γ, when cells were treated with low doses of VCA (4–32 pg mL−1). Another report also confirmed enhanced expression of aforementioned cytokine genes upon stimulation of hPBMC with V. album agglutinin-I.81 These data might suggest new perspective of VCA to regulate the balance between cell proliferation, cytokine production and apoptotic cell death. Induction of these cytokine genes and protein production in the cultures of hPBMC was also observed upon treatment with ML-I.82
VAA extract increased phagocytic activity and candidacidal activity of neutrophils, and decreased adhesion function of epithelial cells. Furthermore, extract stimulated the levels of CD4+CD25+ and CD8+CD25+ T cells and CD3−CD16+CD56+ natural killer cells.83 A study reported that the cell killing capacities of mistletoe extracts are host tree-specific and not correlated with ML or viscotoxin content.84 The newly isolated mistletoe viscotoxins such as VTA1 (85 nm), VTA2 (18 nm) and VTA3 were found to increase natural killer cell-mediated cytotoxicity.85 Impact of the viscotoxins on human granulocytes was studied by flow cytometry and it was found that viscotoxins at 25 and 250 μg mL−1 concentrations enhanced phagocytosis and burst activity against E. coli infection in respiratory track.86 The SC treatment of aqueous mistletoe extract in 8 volunteers was reported to induce the secretion of Th1 (IFN-γ) or Th2 (IL-4) cytokines and also the release of TNF-α and IL-6.87
Numerous studies have reported that Iscador Pini (a fermented extract of mistletoe) induces a strong stimulatory response of hPBMC in normal and allergic individuals. A study was conducted to examine the cell subtypes involved in this in vitro reactivity. Flow cytometry results clearly showed that Iscador activates T cells (CD3+), especially CD4+ T helper cells, as well as monocytes at concentrations of 0.1 to 1.0 mg mL−1. No evidence for a key involvement of B cells (CD19+), NK cells (CD56+), and T suppressor cells/cytotoxic T lymphocytes (CD8+) was detected.88 A recombinant V. album agglutinin (rVAA) enhanced the secretion of an active form of IL-12 and potentiated the cytokine-induced NK cell activation in cultured rat splenocytes. These authors also stated that the effects of rVAA could be associated with its enhancing effects on MHC-unrestricted cytotoxicity in vivo.89 However, the contradictory report on phagocytic activity of lectin is also reported and investigators found that various concentrations of lectin ranging from 0.025 to 20 ng mL−1 had only marginal effect on phagocyte activity.
5.3. Cytotoxicity
ML can induce apoptosis via apoptosis-associated factor-1 (Apaf-1) pathway by stimulating mitochondrial membrane potential (MMP) breakdown and stimulating caspase-3.90,91 The c-Jun N-terminal kinase (JNK) stimulation by ML-I leads to translocation of the pro-apoptotic proteins Bax and Bad. ML-I down regulates B-cell lymphoma 2 (Bcl-2) and up regulates TNF-α and hence provoke apoptosis. We have demonstrated that VA Qu FrF, induces significant cell toxicity in vitro in the human T cell lines CEM and in monocyte cell lines HL-60 and MM-6.92 The viscotoxin-free V. album extract significantly enhanced granulocyte activity and this effect was correlated with the content of the ML.93Treatment of V. album preparations from eight dissimilar host trees (Iscucin Abietis, Pini, Populi, Mali, Salicis, Crataegi, Quercus and Tiliae) showed a significant cytotoxic effect on the medulloblastoma cell lines including Daoy, D342, D425, and UW-288-2, yet the cell susceptibility was unrelated against the different extracts. The reduction in mitochondrial activity and enhancement in apoptotic cell death correlated with the lectin content of the used preparation in a dose-dependent manner.94 ML-I, ML-II, and ML-III were found to be toxic for Molt 4 cells at pg concentrations, ML-III being the most cytotoxic. Interestingly, the digalactosides Gal beta 1,2-Gal beta-allyl and Gal beta 1,3-Gal beta-allyl were able to bind to the B-chain of these lectins and inhibit their toxic activity. N-Acetyl-D-galactosamine and rho-nitrophenyl N-acetylgalactosamine prevented the toxic effects of ML-II and III.95
Major cytotoxic components were fractionated from Korean mistletoe and the changes in their cytotoxic effects due to heat treatment were studied. ML-I showed maximum toxicity, but was disappeared upon heating for 30 min. The study suggested that the ML is not responsible for inducing apoptosis rather it might be due to other components.96 Viscotoxins and alkaloids were found to retain their effects even after heating for 60 and 180 min, respectively. Moreover, the alkaloid fraction was more effective to induce cytotoxic effects on tumor MSV cells than non-tumor A31 cells.97 The isolated KML-C showed strong cytotoxicity against various human and murine tumor cells by inducing apoptosis mediated by Ca2+/Mg2+-dependent endonucleases. However, the cytotoxic activity of KML-C was higher than that of a lectin from European mistletoe V. album spp. loranthaceae.98
5.4. Anti-angiogenic
Treating B16L6 melanoma cells with V. album suppressed tumor growth and resulted in DNA fragmentation and nuclear morphological changes, suggesting that V. album inhibits tumor growth and metastasis by elevating apoptosis and blocking angiogenesis.99 Our group has shown that VA Qu FrF induces apoptosis of endothelial cells in human umbilical vein endothelial cells and in immortalized human venous endothelial cell line.12 Fermented mistletoe extract (FME) treatment of glioblastoma cells down-regulated TGF-β and matrix-metallo-proteinase genes expression, which involve in glioblastoma progression and malignancy. In addition, FME reduced the migratory and invasive potential of glioblastoma cells.100 VAA-I is a plant lectin, which possesses anti-tumoral properties. VAA-I was reported to induce apoptosis in PLB-985 cells and cells from chronic granulomatous disease via caspase-mediated pathway.101The role of VAA-I on activated neutrophils and pro-inflammatory properties have not much explored so far. Lavastre et al. demonstrated that VAA-I at 1000 ng mL−1 activates apoptotic cell death in lipopolysaccharide (LPS)-treated human neutrophils in vitro as well as in murine neutrophils isolated from LPS-induced neutrophil influx.102 They concluded that VAA-I can inhibit LPS-induced pro-inflammatory response in vivo.102 VAA-I induces apoptosis in human neutrophils by accelerating the loss of anti-apoptotic Mcl-1 expression and the degradation of cytoskeletal paxillin and vimentin proteins via caspases.103 Iscador Qu, an aqueous fermented extract from the European mistletoe grown on oaks caused early cell cycle inhibition followed by apoptosis in a dose-dependent manner in endothelial cell cultures. Apoptosis was induced by activating the mitochondrial activity.104
Epi-oleanolic acid, a triterpene was isolated from the dichloromethane extract of Korean V. album (KVA) by repeated silica gel chromatography and recrystallization. Treatment of triterpene showed a typical pattern of apoptotic cell death, including morphological changes and DNA fragmentation in human and murine cancer cells.105 A study on the anti-cancer mechanisms of action of VCA from Korean mistletoe suggested that VCA induces apoptosis in hepatocarcinoma Hep3B cells by inducing ROS production and a loss of DeltaPsim, in which JNK phosphorylation plays a key role in these events.106 The β-galactoside- and N-acetyl-D-galactosamine-specific lectin II, polysaccharides, and viscotoxin of mistletoe were found to induce apoptosis in U937 cells through the activation of phosphotransferase activity in JNK1/stress-activated protein kinase and was characterized by DNA ladder pattern fragmentation.107 However, protein kinase A or C protected the apoptosis induced by MLII of KVA in the human leukemic HL-60 cells.108 The viscotoxins induced cell death by producing mitochondrial Apo2.7 molecules and by generating ROS-intermediates in lymphocytes.109
5.5. Anti-oxidant
It is well known that the anti-oxidant activity of V. album extracts are varied depending on the host tree and the harvesting time. It was observed that extract from the lime tree or white locust tree completely inhibits mitochondrial DNA damage induced by H2O2 in HeLa cells, while extract from hedge maple tree inhibits mitochondrial DNA damage only by 50%.110Organic extracts of V. album, which contains polyphenolic compounds were reported to exert anti-glycation and anti-oxidant properties.38 Oxidative stress protective activity of Korean mistletoe lectin was examined under in vitro system using LLC-PK1 renal epithelial cells. Korean mistletoe lectin exhibited strong 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging potential with an IC50 value of 42.6 μg mL−1.111 In addition, it exerted free radical quenching potential against nitric oxide (NO), superoxide anion, and hydroxyl radical in a concentration-dependent manner. Further, inhibition of COX-2, inducible NO synthase, SIN-1-induced nuclear factor kappa B, and the phosphorylation of inhibitor kappa B alpha was also seen in lectin-treated LLC-PK1 cells.111
Methanol extracts of mistletoe grown on different host trees were studied for their potential anti-oxidant activity. The extract from mistletoe grown on lime tree in summer showed the highest 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging and ant-lipid peroxidation activities.112
5.6. Anti-tumoral
A pre-clinical study suggested that aqueous mistletoe extract exhibits potent anti-tumoral activity by depleting hypoxanthine and activating xanthine oxidase in the cancer cells, which lead to lowered salvage pathway activity required for the cancer cells to proliferate in the cancerous colon tissue.113 Lipophilic extract of V. album and its predominant triterpene oleanolic acid significantly decreased monocyte chemotactic protein-1-induced monocyte transmigration.114 Ethanol extract of V. album containing viscotoxin enhanced the anti-tumor effect of doxorubicin.115 Pretreatment of C6 glioma cells with 100 μg mL−1 of VAE before heat shock significantly decreased expression levels of Hsp27 (73%), 14-3-3β (124%), 14-3-3γ (23%), and 14-3-3ζ (84%) proteins. Increased apoptosis was also observed through caspase-3 activation (60%).116 VAA-I enhanced anti-proliferating potential of cycloheximide in the human lung carcinoma cell line A549 by inducing G1-phase accumulation.117 The KML-II was also found to induce apoptosis in U937 cells via activation of caspase cascades.118A large number of studies on synergistic effect of mistletoe extract and its components are available. IFN-γ enhanced the apoptotic response to ML-II through augmentation of Fas/Fas L expression and caspase activation in human myeloid U937 cells.119 The MLs activated apoptotic pathway in various tumour cell lines and human lymphocytes. The ML-III was also found to reduce the expression of nuclear p53 and Bcl-2.120
Oleanolic acid, a component of the leaves and roots of V. album induced apoptosis by altering cellular morphology as well as DNA integrity in HaCaT cells in a dose-dependent manner, with comparatively low cytotoxicity.121 Either solubilized triterpene acids or lectins and combinations thereof were found to induce dose-dependent apoptosis in the acute lymphoblastic leukaemia cell line NALM-6 via caspase-8 and -9 dependent pathways in vitro and in vivo.122 V. album from apple and pine increased anti-tumoral activity of activated human macrophages by inducing the production of NO.123 Anti-tumor activity of Iscador M Spezial, Iscador Qu Spezial and Iscador P preparations of V. album at high concentrations were investigated in a panel of 12 cell lines.124,125 The lectin-containing Iscador M Spezial and Iscador Qu Spezial showed a noticeable anti-tumor activity in the mammary MAXF cancer 401NL cells at 15 μg mL−1 concentration with more than 70% growth inhibition compared to untreated control cells.124,125
Anti-proliferative effect of V. album extracts was characterized in the bladder carcinoma T24, TCCSUP, J82 and UM-UC3 cell lines. Necrosis and apoptotic cell death were the fundamental mechanisms of anti-tumoral effect of V. album extracts.126 The primary structure and anti-tumor activity of a novel peptide from stem and leaves of mistletoe (V. coloratum (Kom.) Nakai) was examined on the rat osteoblast-like sarcoma 17/2.8 cells.127 The primary structure of the peptide named as viscotoxin B2 was KSCCKNTTGRNIYNTCRFAGGSRERCAKLSGCKIISASTCPSDYPK and its IC50 value was 1.6 mg L−1. Viscin, betulinic acid, oleanolic acid and ursolic acid, which are lipophilic compounds of V. album were found to inhibit growth and induce apoptotic cell death in Molt4, K562 and U937 leukaemia cells. However, the growth inhibitory effect of viscin was more prominent on Molt4 and U937 cells with IC50 values 118 ± 24 and 138 ± 24 μg mL−1 respectively.48
VCA has been shown to induce apoptosis by decreasing Bcl-2 level and telomerase activity and by inducing of Bax through p53- and p21-independent pathway in hepatoma cells. Later on, the induction of apoptosis via activation of caspase-3 and the inhibition of telomerase activity through transcriptional suppression of hTERT in the VCA-treated A253 cells was reported.128 Treatment with VCA also induced apoptosis in both SK-Hep-1 (+p53) and Hep 3B (−p53) cells through p53- and p21-independent pathways. Apoptosis induction was related to down-regulation of Bcl-2 and up-regulation of Bax functioning upstream of caspase-3. Moreover, VCA caused down-regulation of telomerase activity in both the cells.91 Signaling through lectins could involve modulation of protein kinase activities. However, the alpha/beta-galactoside-binding lectins, isolated from mistletoe leaves, did not inhibit the epidermal growth factor receptor tyrosine kinase activity of rat liver.129
Administration of lectin (KML-C) from Korean mistletoe (20–50 ng per mouse) for 2 days by intravenous route before tumor implantation significantly reduced lung metastases of B16-BL6 and colon 26-M3.1 cells. Importantly, KML-C treatment one-day post-tumor implantation not only significantly suppressed lung metastasis of B16-BL6 and 26-M3.1 cells, but also reduced liver and spleen metastasis of L5178Y-ML25 lymphoma cells. Mechanistically, it was found that KML-C treatment (50 ng per mouse) for 2 days significantly increased NK cell-mediated cytotoxicity against tumor cells and tumoricidal activity of peritoneal macrophages.130 Similar results were also observed with Korean mistletoe extract KM-110, wherein administration of KM-110 (100 μg per mouse) for 2 days by intravenous route before tumor implantation significantly blocked lung metastases of B16-BL6 and 26-M3.1 cells, and liver and spleen metastasis of L5178Y-ML25 cells. This effect on tumor metastasis was also mediated by NK cell activation.131 Additionally, multiple administration of KM-110 into tumour-bearing mice resulted in significant inhibition of primary tumour growth.131 Administration of the Iscador (1 mg Iscador per dose, IP) inhibited lung metastasis of B16F10 melanoma cells in mice by reducing nodule formation (92%) and enhanced the life-span (71%) of animals.132 The IC50 was found to be 0.0166 mg per dose. However, galactoside-specific mistletoe lectin failed to inhibit N-methyl-N-nitrosourea-induced tumor development in the urinary bladder of rats and to mediate a local cellular immune response following long-term administration.133 Therefore, anti-tumoral functions are not mediated by all lectin preparations of mistletoe.
Intravenous treatment with a standardized mistletoe extract at 3, 30 or 150 ng kg−1 doses once daily for 3 weeks exerted inhibitory effects (58 to 95%) on the lung metastasis of B16. A significant reduction in the percentage of bronchoalveolar lavage pigmented cells was also noticed.134 The study conducted by Srdic-Rajic and co-workers investigated synergistic anti-tumor effects of V.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) album and doxorubicin chemotherapeutic agent on chemo-resistant chronic myelogenic leukemia K562 cells. Authors found that V.
album and doxorubicin chemotherapeutic agent on chemo-resistant chronic myelogenic leukemia K562 cells. Authors found that V.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) album enhances the anti-leukemic efficiency of doxorubicin against chemo-resistant K562 cells by checking the G2/M arrest and by stimulating apoptosis.135
album enhances the anti-leukemic efficiency of doxorubicin against chemo-resistant K562 cells by checking the G2/M arrest and by stimulating apoptosis.135
5.7. Anti-diabetic
V. album has been well-documented as a traditional treatment for diabetes. The Korean mistletoe V. album var. coloratum enhanced the insulin secretion from pancreatic β-cells without any cytotoxicity effects. Moreover, upregulated pattern of insulin genes such as PDX-1 and β2/neuroD was also observed in V. album var. coloratum-treated mice. Thus, VAC could be considered as a useful source for the development of anti-diabetic drug to reduce the blood glucose level of type I diabetic patients.136 Structural analysis of ML-1 complexed with galactose and lactose revealed unique sugar binding abilities.137 Among the medicinal plants, V. album showed potent alpha-glucosidase inhibitory activity.138 The aqueous extract of mistletoe (1–10 mg mL−1) was reported to stimulate secretion of insulin (1.1- to 12.2-fold) from clonal pancreatic β-cells. The ability of extract to enhance insulin secretion was not mediated by lectins.139 The results indicated the presence of insulin-releasing natural product(s), which might contribute to the reported anti-diabetic property of the mistletoe.5.8. Anti-hypertensive
Acute effect of different extracts of mistletoe stem on arterial blood pressure was studied in Wistar rats.140 The ethanol extract showed the superlative effect even at the lowest applied concentration (3.33 × 10−5 mg kg−1) and significantly reduced the blood pressure after applied concentration (1.00 × 10−3 mg kg−1). However, other extracts such as ether and ethyl acetate showed the activity only at higher concentrations.5.9. Anti-microbial
Methanol extract of V. album showed anti-microbial activity against 9 out of 32 pathogenic microorganisms.141 Different extracts from the leaves of V. album L. ssp. album were prepared and analyzed for their effect on human parainfluenza virus type 2 (HPIV-2) growth in Vero cells.142 The aqueous extract (1 μg mL−1) was found to prevent HPIV-2 replication and that virus production was inhibited >99% without any toxic effect on host cells. This activity could neither be credited to the direct HPIV-2 inactivation nor to the inhibition of adsorption to Vero cells. Five patients with chronic hepatitis C showed 6–20% reduction in the viral load and normalization of liver inflammation (two patients) without side effects following treatment with Iscador for one year.143 Two other patients were also in complete remission of their elevated aspartate transaminase and alanine transaminase. However, IFN-γ increase in the serum of HIV-positive and healthy subjects was not noticed following subcutaneous injection of a non-fermented V. album extracts.1445.10. Anti-mutagenic
V. album var. coloratum was evaluated for its anti-mutagenic activity against the mutagens such as 2-aminoanthracene (2AA) and furylfuramide-2 for Salmonella typhimurium strain TA98, and sodium azide (NaN3) and 2AA for S. typhimurium strain TA100 using Ames test. V. album var. coloratum was more effective in preventing the mutagenicity of the indirect-acting mutagen 2-AA, when tested with both the strains.1455.11. Miscellaneous properties
European mistletoe is known for its anti-cancer and immune enhancing activities but few data exist on anti-convulsant activity. Treatment with V. album managed refractory childhood absence epilepsy in a 4.5 year old girl.146 V. album lipophilic extract (10 μg mL−1) and its oleanolic acid (1 μg mL−1) have shown excellent wound healing activity. It was associated with the stimulation of migration of NIH/3T3 fibroblasts.147 Administration of V. album var. coloratum (50 μg mL−1) increased the mean survival time by 9.61 and 19.86% in Caenorhabditis elegans and Drosophila melanogaster, respectively.148 Treatment with V. album var. coloratum extract (3 g per kg per day) had an anti-obesity effect and protected against hepatic steatosis in mice with high-fat diet-induced obesity. The effects appear to be mediated through an increased mitochondrial activity.149 KME was reported to induce mitochondrial activity possibly by activating PGC-1α and SIRT1, and improved the endurance of mice. Authors have suggested that KME could be used as a novel mitochondria-activating agent.150 A pre-clinical study suggested that aqueous extract of V. album leaves exhibits sedative, anti-epileptic and anti-psychotic activities in mice and rats.2To find out the promising pancreatic lipase (triacylglycerol acylhydrolase) suppressors from natural products, 61 medicinal plants from Korea were tested for their anti-lipase activity for the prevention of obesity. The V. album extracts showed anti-lipase activity with IC50 values of 33.3 μg mL−1 and 35.15 μg mL−1 for anti-phosphodiesterase.151 Aqueous extract of V. album decreased the serum cholesterol, and HDL-cholesterol, triglyceride concentrations in the mice fed with high-cholesterol diet without inducing any gastric damage, suggesting potent hypocholesterolaemic activity.152 The aqueous extract of V. album leaves exhibited a significant coronary vasodilator activity in the Langendorff's isolated perfused heart model. Authors have also suggested that extract contains some bioactivity constituents that may act as inducers of the NO/soluble guanylate cyclase pathway.153 Formation of lactose-resistant aggregates of human platelets and differential signaling responses to cell contact formation by ML were also been reported.154
6. Clinical trials
Although mistletoe preparations are currently being used in different clinical settings, the most important clinical use has been in the field of cancer as a complementary therapy to reduce the adverse reactions of conventional chemotherapies. In fact, mistletoe extract therapy is among the most thoroughly studied complementary treatments in Europe. Several systematic reviews and meta-analysis have found a benefit from mistletoe treatment in cancer patients and in minimizing the side effects of anti-cancer chemotherapy.155–159 However, these reviews have also found that nearly all studies suffered from methodological shortcomings to some degree and many of the studies were not conclusive. Earlier review had found that even statistical pooling is not possible because of the heterogeneity of the primary studies,160 therefore only a narrative systematic review was conducted.Furthermore, a Cochrane review was done with the objective to determine the effectiveness, tolerability and safety of mistletoe extracts either as a monotherapy or administered as an adjunct to conventional cancer treatment.161 Cochrane reviewers have found that from 80 mistletoe studies examined for the purpose of assessing mistletoe therapy in oncology, 58 had no prospective trial design with randomized treatment allocation and were excluded from the analysis. Although 6 trials among 13 that investigated survival upon mistletoe therapy showed certain evidence of therapeutic benefit, none of them met with high methodological quality. Among 16 trials that explored the efficacy of mistletoe extracts for either improved quality of life (QOL), psychological parameters, performance index, symptom scales or the reduction of adverse effects of chemotherapy, only 2 of them were of a superior methodological quality.161 Thus, the overall conclusion was that independent clinical research of superior quality is required to accurately assess the safety and therapeutic effects of mistletoe extracts.
A study published by Gerhard et al. in 2004 illustrated the difficulties in enrolment and randomization of cancer patients for the therapy with mistletoe.162 Among 1922 patients who were operated for breast tumor, 154 patients who met the inclusion criteria agreed to participate in the study. However, 80 patients were subsequently excluded from the study following evaluation of the final results on tumor staging and conventional treatment plan. This study suggested that only 29 (39%) of the remaining 74 patients would have agreed to participate in a randomized trial on mistletoe therapy for breast cancer. However, several randomized clinical trials assessing safety and effectiveness of mistletoe preparations have been published in recent years providing clear evidence for improved survival and QOL of cancer patients treated with mistletoe preparations.
6.1. Pancreatic cancer
Two hundred and twenty patients with inoperable or metastatic pancreatic cancer were included in a prospective randomized clinical trial. Hundred and ten patients received Iscador® and remaining 110 patients received no anti-neoplastic therapy. Patients treated with Iscador® survived better (4.8 months) than the control group (2.7 months) (HR = 0.55; p = 0.0031). Importantly, no therapy-related adverse events reported in the Iscador group. V. album therapy thus exhibited a significant and clinically-relevant prolongation of overall survival.163 In the same single-center, group-sequential, randomized phase III trial (ISRCTN70760582),164 data on QOL and body weight were obtained from 96 patients treated with mistletoe and 72 control patients. Patients treated with mistletoe performed better on all the 6 functional scales and on 7 of the 9 symptom scales European Organisation for Research and Treatment of Cancer QOL questionnaire (EORTC QLQ-C30), including pain (95% confidence interval [CI] −29 to −17), fatigue (95% CI −36.1 to −25.0), appetite loss (95% CI −51 to −36.7) and insomnia (95% CI −45.8 to −28.6). This was reflected by the body weight trend of the patients during the study period. The results indicated that mistletoe treatment significantly improves the QOL in comparison to best supportive care alone.164 The study suggested that V. album is non-toxic and effective second-line therapy for patients with locally advanced or metastatic pancreatic cancer.6.2. Breast cancer
A prospective randomized open label pilot study on 95 breast cancer patients showed an improvement of QOL when treated with a combination of chemotherapeutic agents cyclophosphamide, adriamycin and 5-fluoro-uracil (CAF), and Iscador® M special (IMS). The control group received only CAF.165 A descriptive analysis of all 15 scores of the EORTC-QLQ-C30 displayed better QOL in the IMS group compared to the control group. Significant differences were observed among 12 scores (p < 0.02) and clinically-pertinent and significant difference of minimum 5 points were noticed in nine scores. IMS group showed a trend of lower frequency of CAF-induced neutropenia. This pilot study thus showed the importance of IMS to improve the QOL of the patients treated with CAF. A five-year follow-up study suggested that adding V. album during chemotherapy of early stage breast cancer patients does not influence the frequency of relapse or metastasis within 5 years.1666.3. Bone cancer
A recent randomized study investigated post second metastatic relapse (12 month) disease-free survival rate in osteosarcoma patients following treatment with Viscum sc or oral Etoposide (a topoisomerase inhibitor anticancer drug). Twenty patients with a median age of 34 years (ranging 11–65 years) were enrolled and were treated randomly with Viscum sc or oral Etoposide. Patients were monitored for a median follow-up time of 38.5 months (3–73). The median postrelapse disease-free survival in the oral Etoposide was 4 months (1–47) and it was 39 months (2–73) in the Viscum group. Also, because of lower toxicity, Viscum-treated patients reported a higher QOL.167 However, authors have also suggested that a larger study is obligatory for the firm determination of the efficacy and immunomodulatory mechanisms of Viscum therapy in osteosarcoma.6.4. Lung cancer
A randomized phase II study was conducted in chemotherapy-naïve advanced non-small-cell lung cancer (NSCLC) patients to evaluate the influence of Iscador therapy on carboplatin-containing treatments-related side-effects and QOL. Seventy-two patients were registered for this study with 39 patients for control and 33 for Iscador. Majority of the patients (65%) were in stage IV and had squamous histology (62%). Iscador therapy did not modify the overall survival of the patients and median overall survival was 11 months in both the groups. Although not significant, Iscador group showed a tendency of higher time to tumour progression (TTP). Median TTP was 4.8 months for the controls and 6 months in the Iscador. Grade 3–4 hematological toxicities were similar between both the groups. However, patients in the control group had significantly higher chemotherapy dose reductions (44% vs. 13%, p = 0.005), grade 3–4 non-hematological toxicities (41% vs. 16%, p = 0.043) and hospitalizations (54% vs. 24%, p = 0.016), suggesting that Iscador reduces the chemotherapy-related toxicity. Additional clinical trials are required to confirm and validate these results.168A multi-center randomized open prospective clinical trial was conducted to assess the impact of standardized mistletoe extract (sME) therapy on QOL in various types of cancers.169 The study enrolled 233 patients with NSCL (n = 94), breast (n = 68) and ovarian (n = 71). The 224 patients who fulfilled all the criteria were grouped into two. One hundred and fifteen patients were treated with sME HELIXOR A and 109 control group patients were treated with the approved immunomodulating phytopharmacon Lentinan. All the patients with treated with sME or Lentinan complimentary therapy during chemotherapy regimen. QOL was determined by the Functional Living Index-Cance, Traditional Chinese Medicine Index and the Karnofsky Performance Index. Authors found that patients complementarily treated with sME had significantly improved QOL (p < 0.05) as compared to control group. Adverse effects were also less frequent and self-limiting in sME-treated patients.169 This trial suggested that complementary sME therapy can improve the QOL in cancer patients by reducing the side-effects of chemotherapy.
6.5. Advanced solid tumors
The phase I study of gemcitabine (GEM) and V. album in patients with advanced solid cancers (ASC) was conducted for the evaluation of safety, toxicity, and maximum tolerated dose (MTD); absolute neutrophil count (ANC) recovery; formation of mistletoe lectin antibodies (ML Abs); plasma cytokine concentrations; clinical response and pharmacokinetics of GEM.170 Forty four patients with advanced pancreatic, NSCLC, recurrent metastatic colorectal or breast cancer was included. In the first stage, increasing dose of V. album and fixed dose GEM was used. In the second stage, increasing dose of GEM and fixed dose of V. album was used. This study found that all the patients showed immune response to mistletoe injections as determined by ML3 IgG Abs. Compliance with mistletoe therapy was high and the median survival was 200 days with partial response in 6% patients and stable disease in 42%. Dose-limiting toxicities attributed to V. album were G4 neutropenia, G4 thrombocytopenia, G4 acute renal failure, and G3 cellulitis. MTD was GEM 1300 mg m−2 and mistletoe 250 mg combined and V. album did not affect pharmacokinetics of GEM. This study indicated that combined GEM and V. album is well tolerated by patients with advanced sold tumors. Clinical response in the group received combination therapy was similar to GEM alone treated patients.6.6. Cancer-related fatigue
Although not examined in a randomized clinical trial, the use of mistletoe preparations has shown an improvement of cancer-related fatigue (CRF).171 The fatigue levels among 324 patients with non-metastasized colorectal cancer (Union for International Cancer Control stage I–III) during the first-line chemo- or radio-chemotherapy protocols were assessed. Iscador(®) Qu was given to 181 patients compared to control group of 143 patients without this supportive care treatment. At the end of the median treatment period, CRF was diagnosed in 16 patients (8.8%) treated with Iscador(®) Qu and was 60.1% in chemo- or radio-chemotherapy group without Iscador(®) Qu. Multivariable-adjusted OR = 10.651 (95% CI 5.09–22.28; p < 0.001) at the first visit was dropped to OR = 0.054 (95 CI 0.02–0.13; p < 0.001) at the end of therapy.7. Toxicological studies
For the development of remedies in general, knowledge on the toxicology is crucial to confirm drug safety. Cancer patients who received SC injections of mistletoe extracts were examined for adverse drug reactions (ADRs). Out of 1923 patients, 14.7% patients reported local reactions less than 5 cm and raised body temperature less than 38 °C. Among 162 patients who reported ADRs, these reactions were mild (50.8%) to moderate (45.1%) in majority of the patients. Only 4.2% patients reported severe ADR. There were no recognizable risk factors for ADRs. The ADR rate augmented as the dose of mistletoe increased. However, patients receiving concurrent conventional therapies reported less ADRs during mistletoe therapy. The study indicated that mistletoe therapy is safe.172 In another report, SC injections of ML-1 (1 mg kg−1, weekly twice) for a month period resulted in significant enhancement of several acute phase reactants including C-reactive protein, haptoglobin and complement component C3.173 Viscotoxins were also shown to stimulate the generation of reactive oxygen species in human lymphocytes, as well as cell death.174 Altogether, mistletoe preparations at high doses were reported to cause hypotension, pupil contraction, vomiting, intestinal cramps, diarrhea and seizures. In addition to pain and irritation at the site of injection, SC route of administration of mistletoe can also trigger mild to severe headaches, chills, angina, fever and allergic reactions.Experimental studies have shown that effects of mistletoe viscotoxins and phoratoxin on the circulation were responsible for the reflex bradycardia, negative inotropic effects and vasoconstriction observed in the cardiac muscles of cats.21 In addition, viscotoxins reduced isometric twitch and caused contracture and progressive depolarization in rabbit heart preparations.21,175 It was also proposed that phenylpropanoids might mediate cardiovascular effects by suppressing cAMP phosphodiesterase.176 Therefore, use of mistletoe in patients with cardiovascular diseases requires caution. A systemic review on the safety of mistletoe in animals and humans revealed that this therapy is not associated with immunosuppression. The side effects were mostly dose-dependent flu-like symptoms, local reactions at the site of injection of mistletoe and miscellaneous mild effects. Some reports of allergic reactions and reversible hepatotoxicity with high doses of recombinant ML were also recorded.177
Toxicity of oral mistletoe exposure is controversially discussed. In 1952, Winterfeld stated that oral application of powdered V. album extracts or drops were well tolerated and did never induced toxic reactions.178 Further, it was also observed that consumption of berries up to three or one to two leaves of American mistletoe Phoradendron serotinum (Loranthaceae) seems unlikely to cause severe toxicity.179 However, consumption of a herbal product containing mistletoe as one of the ingredients caused hepatitis in a women.180 However, the role of mistletoe was not proved in this case. Weeks and Proper also reported a case of chronic active hepatitis after ingestion of a herbal remedy containing mistletoe, skullcap, valerian and other plants. Again, this study could not prove mistletoe as an underlying cause.181
Toxicity of Iscador and a purified protein fraction of V. album by parenteral route were examined in vivo in mice.182 Authors could observe long-term toxicity only with the purified but not well-characterized proteins. Mortality accompanying with liver atrophy and other organs involved in metabolism, and thymus disintegration was recorded 3–4 days post V. album injection. However, 5–10% of the LD50 concentrations of these proteins caused enlarged spleen and thymus. However, precise concentration of the ingredients in the injected preparation was not known in this study. Subsequent study by Rentea and colleagues tried to determine the LD50 of a Iscador by using precise concentration of the product.183 They found LD50 dose following IP injection varies among different strains and species of the animals. Thus, LD50 was 700 mg kg−1 for CD-1 outbred albino mice; 348 mg kg−1 for C57/BL6 mice and 378 mg kg−1 for Sprague-Dwaley rats. These animals at lethal doses showed hemorrhagic peritonitis and died with tonic and clonic seizures. On the other hand, LD50 of VA-E (Iscador Mali) in mice was lower (168 mg kg−1).184 However, LD50 of VA-E (Iscador Quercus) in mice was at higher range: 500 mg kg−1 by intravenous route and 1200 mg kg−1 by SC route.185 Studies in the animals did not give any indications on the adverse effects of mistletoe on the reproduction and genotoxic effects.177
Administration of high dose VA-E (Lektinol) at 100 mg kg−1 caused mortality of all the rats within 5 min.186 These animals experienced dyspnea, ataxia, sedation, exophthalmos and spasms. However at 25 mg kg−1, animals showed dyspnea and sedation with no death. The sub-chronic toxic doses of 0.2, 1.5 and 5 mg kg−1 for 4 weeks did not reveal any organ toxicity.186
Toxic effects of purified components of mistletoe were also been explored. In mice, the LD50 of ML-1 was found to be 80 μg kg−1.187 Another report suggested that LD50 of ML-1 and ML-3 were 28 and 49 mg kg−1, respectively.185 But the lectin activity and route of application were not clear in these reports. Subsequent study however reported lower LD50 values when different lectins were injected by IP route: ML-1: 28 μg kg−1, ML-2: 1.5 μg kg−1 and ML-3: 55 μg kg−1.24 ML-1 in rat was lethal within 24 hours at 100 μg kg−1 by IP route. However, 10 μg kg−1 caused mortality in 3–4 days.188 These animals experienced pancreatic hemorrhages, ascites and congested intestine, and these symptoms were similar to those observed with ricin. Thus, toxicological values for ML preparations showed large variations probably due to differences in the methods that calculate lectin activity. High production of TNFα and hemagglutinating activity of the lectins were proposed as underlying mechanisms of ML toxicity.189
8. Conclusions and perspectives
V. album, a plant that has been described from mythological times as a potent remedy for several pathologies continues to evoke interest and scientific curiosity among researchers. Even after a century after its introduction as a treatment for cancer, the clinical use as a component of supportive care, and knowledge on the mechanisms of action of mistletoe continue to expand. Over hundred clinical studies have provided evidence in support of the beneficial effects of mistletoe in cancer patients and mistletoe thus remains as one of the complementary remedies most often used. The results from several randomized clinical trials suggested that mistletoe preparations are safe and improved overall survival and QOL of cancer patients.Our essay is focused on the active components of mistletoe extracts and pluripotent biological activities. A wide spectrum of pharmacologically active metabolites that belong to a variety of chemical entities of proteins, polysaccharides, liposoluble compounds and secondary metabolites have been identified in V. album extracts. It is conceivable that the heterogenous profile of biochemical compounds provides the basis to the broad diversity of pharmacological activities of mistletoe as each single component contributes diverse modes of actions in addition to imparting to a synergistic beneficial action in conjunction with other molecules. Although a large number of the anti-tumoral properties of mistletoe preparations have been attributed to the lectins, it is possible that enlarging the scope of research to other components especially polyphenols would open new perspectives.
Although a number of elegant pre-clinical studies and numerous powerful clinical trials have provided ample lines of evidence in favor of potent anti-cancer activity of mistletoe if used as concomitant therapeutics in parallel to standard therapy, the field is plagued by a certain degree of skepticism. This may be due to homeopathic origin of the therapy, or inconclusive beneficial effects in Cochrane review or highly scattered technically-sound scientific reports of exploring the molecular and cellular mechanisms underlying the beneficial effects of mistletoe in cancer patients. Thus, further studies examining the results of V. album extracts in the adjunct therapy of cancer should aim at evidence-based clinical data, superior quality, transparent study-design and clear end-points to deliver higher perceptiveness into a supportive therapy that is frequently disapproved as ineffective. Such careful analysis of the effects of V. album should help in clarifying certain skepticism clouding over its use, and provide more effective pointers to the clinicians in adopting appropriate treatment regimes.
Abbreviations
| 2AA | 2-Aminoanthracene |
| ADRs | Adverse drug reactions |
| Aps | Arabinogalactan proteins |
| Bcl-2 | B-Cell lymphoma 2 |
| Cb | Chitin-binding |
| COX-2 | Cyclooxygenase |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FME | Fermented mistletoe extract |
| HPIV-2 | Human parainfluenza virus type 2 |
| HUVEC | Human umbilical vein endothelial cells |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| JNK | c-jun N-terminal kinase |
| KME | Korean mistletoe extract |
| KML | Korean mistletoe lectin |
| KML-C | Lectins from Korean V. album spp. coloratum |
| KVA | Korean V. album |
| LPS | Lipopolysaccharide |
| ML | Mistletoe lectin |
| NK | Natural killer |
| NO | Nitric oxide |
| QOL | Quality of life |
| rVAA | Recombinant V. album agglutinin, TNF-α, tumor necrosis factor-alpha |
| VAA | V. album ssp. album |
| VAA-I | V. album agglutinin-I |
| VAC | V. album var. coloratum |
| VAE | Viscum album extract |
| VCA | V. album var. coloratum agglutinin |
| VF-2 | Viscum fraxini-2 |
References
- M. L. Steele, J. Axtner, A. Happe, M. Kroz, H. Matthes and F. Schad, Integr. Cancer Ther., 2015, 14, 140–148 CrossRef PubMed.
- G. Gupta, I. Kazmi, M. Afzal, M. Rahman, S. Saleem, M. S. Ashraf, M. J. Khusroo, K. Nazeer, S. Ahmed, M. Mujeeb, Z. Ahmed and F. Anwar, J. Ethnopharmacol., 2012, 141, 810–816 CrossRef CAS PubMed.
- O. E. Ofem, A. E. Eno, C. O. Nku and A. B. Antai, J. Ethnopharmacol., 2009, 126, 421–426 CrossRef PubMed.
- C. Saha, P. Hegde, A. Friboulet, J. Bayry and S. V. Kaveri, PLoS One, 2015, 10, e0114965 CrossRef PubMed.
- D. D. Orhan, M. Aslan, N. Sendogdu, F. Ergun and E. Yesilada, J. Ethnopharmacol., 2005, 98, 95–102 CrossRef PubMed.
- P. Hegde, M. S. Maddur, A. Friboulet, J. Bayry and S. V. Kaveri, PLoS One, 2011, 6, e26312 CrossRef CAS PubMed.
- R. J. Sturgeon, in Carbohydrate Chemistry, ed. J. F. Kennedy and N. R. Williams, The Royal Society of Chemistry, 1981, vol. 12, pp. 287–373 Search PubMed.
- F. Marcelo, F. J. Canada, S. Andre, C. Colombo, F. Doro, H.-J. Gabius, A. Bernardi and J. Jimenez-Barbero, Org. Biomol. Chem., 2012, 10, 5916–5923 Search PubMed.
- M. O. Agbo, D. Lai, F. B. Okoye, P. O. Osadebe and P. Proksch, Fitoterapia, 2013, 86, 78–83 CrossRef CAS PubMed.
- F. Ntie-Kang, L. L. Lifongo, C. V. Simoben, S. B. Babiaka, W. Sippl and L. M. Mbaze, RSC Adv., 2014, 4, 35348–35370 RSC.
- J. P. Duong Van Huyen, J. Bayry, S. Delignat, A. T. Gaston, O. Michel, P. Bruneval, M. D. Kazatchkine, A. Nicoletti and S. V. Kaveri, Mol. Med., 2002, 8, 600–606 Search PubMed.
- S. R. Elluru, J. P. Duong Van Huyen, S. Delignat, F. Prost, D. Heudes, M. D. Kazatchkine, A. Friboulet and S. V. Kaveri, Anticancer Res., 2009, 29, 2945–2950 Search PubMed.
- H. Becker, Oncology, 1986, 43(suppl. 1), 2–7 CrossRef PubMed.
- A. Büssing, Mistletoe: the genus Viscum, 2003 Search PubMed.
- M. Giudici, R. Pascual, L. de la Canal, K. Pfuller, U. Pfuller and J. Villalain, Biophys. J., 2003, 85, 971–981 CrossRef CAS PubMed.
- J. Bogomolovas, B. Simon, M. Sattler and G. Stier, Protein Expression Purif., 2009, 64, 16–23 CrossRef CAS PubMed.
- J. Konopa, J. M. Woynarowski and M. Lewandowska-Gumieniak, Z. Physiol. Chem., 1980, 361, 1525–1533 CrossRef CAS.
- J. Nazaruk and P. Orlikowski, Nat. Prod. Res., 2015, 1–13 Search PubMed.
- J. E. Debreczeni, B. Girmann, A. Zeeck, R. Kratzner and G. M. Sheldrick, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2003, 59, 2125–2132 CrossRef.
- G. Schaller, K. Urech, G. Grazi and M. Giannattasio, Planta Med., 1998, 64, 677–678 CrossRef CAS PubMed.
- S. Rosell and G. Samuelsson, Toxicon, 1966, 4, 107–110 CrossRef CAS PubMed.
- W. J. Peumans, P. Verhaert, U. Pfuller and E. J. Van Damme, FEBS Lett., 1996, 396, 261–265 CrossRef CAS PubMed.
- S. S. Komath, M. Kavitha and M. J. Swamy, Org. Biomol. Chem., 2006, 4, 973–988 Search PubMed.
- H. Franz, P. Ziska and A. Kindt, Biochem. J., 1981, 195, 481–484 CrossRef CAS PubMed.
- T. Hajto, K. Hostanska and H. J. Gabius, Cancer Res., 1989, 49, 4803–4808 CAS.
- R. Wacker, S. Stoeva, K. Pfuller, U. Pfuller and W. Voelter, J. Pept. Sci., 2004, 10, 138–148 CrossRef CAS PubMed.
- R. Krauspenhaar, S. Eschenburg, M. Perbandt, V. Kornilov, N. Konareva, I. Mikailova, S. Stoeva, R. Wacker, T. Maier, T. Singh, A. Mikhailov, W. Voelter and C. Betzel, Biochem. Biophys. Res. Commun., 1999, 257, 418–424 CrossRef CAS PubMed.
- U. Edlund, A. Hensel, D. Frose, U. Pfuller and A. Scheffler, Arzneimittelforschung, 2000, 50, 645–651 CAS.
- B. Amer, O. J. Juvik, G. W. Francis and T. Fossen, Pharm Biol, 2013, 51, 981–986 CrossRef CAS PubMed.
- N. Lannoo and E. J. M. Van Damme, Front. Recent Dev. Plant Sci., 2014, 5, 397 Search PubMed.
- M. Luczkiewicz, W. Cisowski, P. Kaiser, R. Ochocka and A. Piotrowski, Acta Pol. Pharm., 2001, 58, 373–379 CAS.
- A. U. Turker, A. B. Yýldýrým and F. P. Karakas, Spatula DD, 2012, 2, 229–236 CrossRef.
- B. Amer, O. J. Juvik, F. Dupont, G. W. Francis and T. Fossen, Phytochem. Lett., 2012, 5, 677–681 CrossRef CAS.
- D. D. Orhan, I. Çalis and F. Ergun, Pharm. Biol., 2002, 40, 380–383 CrossRef CAS.
- G. S. Kienle and H. Kiene, Integr. Cancer Ther., 2010, 9, 142–157 CrossRef PubMed.
- H. Wagner, E. Jordan and B. Feil, Oncology, 1986, 43(suppl. 1), 16–22 CrossRef CAS PubMed.
- K. Haas, M. Bauer and E. Wollenweber, in Zeitschrift für Naturforschung C, 2003, vol. 58, p. 464 Search PubMed.
- M. I. Choudhary, S. Maher, A. Begum, A. Abbaskhan, S. Ali and A. Khan, Chem. Pharm. Bull., 2010, 58, 980–982 CrossRef CAS PubMed.
- N. X. Nhiem, P. V. Kiem, C. V. Minh, N. Kim, S. Park, H. Y. Lee, E. S. Kim, Y. H. Kim, S. Kim, Y. S. Koh and S. H. Kim, J. Nat. Prod., 2013, 76, 495–502 CrossRef CAS PubMed.
- A. Panossian, A. Kocharian, K. Matinian, E. Amroyan, E. Gabrielian, C. Mayr and H. Wagner, Phytomedicine, 1998, 5, 11–17 CrossRef CAS PubMed.
- H. Wagner, B. Feil, O. Seligmann, J. Petricic and Z. Kalogjera, Planta Med., 1986, 102–104 CrossRef CAS.
- N. X. Nhiem, H. Y. Lee, N. Y. Kim, S. J. Park, E. S. Kim, J. E. Han, H. Yang and S. H. Kim, Magn. Reson. Chem., 2012, 50, 772–777 CrossRef PubMed.
- D. Deliorman, I. Çaliş, F. Ergun and U. Tamer, J. Liq. Chromatogr. Relat. Technol., 1999, 22, 3101–3114 CrossRef CAS.
- K. Tyszczuk-Rotko, K. Domanska, I. Sadok, M. Wojciak-Kosior and I. Sowa, Anal. Methods, 2015, 7, 9435–9441 RSC.
- E. O. Omeje, P. O. Osadebe, C. O. Esimone, C. S. Nworu, A. Kawamura and P. Proksch, Nat. Prod. Res., 2012, 26, 1775–1781 CrossRef CAS PubMed.
- S. Jäger, H. Trojan, T. Kopp, M. Laszczyk and A. Scheffler, Molecules, 2009, 14, 2016 CrossRef PubMed.
- S. Jager, K. Winkler, U. Pfuller and A. Scheffler, Planta Med., 2007, 73, 157–162 CrossRef PubMed.
- K. Urech, J. M. Scher, K. Hostanska and H. Becker, J. Pharm. Pharmacol., 2005, 57, 101–109 CrossRef CAS PubMed.
- D. Deliorman Orhan and I. Orhan, Chem. Nat. Compd., 2006, 42, 641–644 CrossRef CAS.
- T. Cebovic, S. Spasic and M. Popovic, Phytother. Res., 2008, 22, 1097–1103 CrossRef CAS PubMed.
- C. M. Struh, S. Jager, C. M. Schempp, A. Scheffler and S. F. Martin, Phytother. Res., 2012, 26, 1507–1512 CrossRef CAS PubMed.
- H. D. Umucalilar, N. Gulsen, B. Coskun, A. Hayirli and H. Dural, Agroforestry Systems, 2007, 71, 77–87 CrossRef.
- C. N. Ishiwu, J. E. Obiegbuna and N. M. Aniagolu, Niger. Food J., 2013, 31, 1–7 CrossRef.
- T. A. Khwaja, J. C. Varven, S. Pentecost and H. Pande, Experientia, 1980, 36, 599–600 CrossRef CAS PubMed.
- K.-W. Kim, S.-H. Yang and J.-B. Kim, Evid. Based Complement Alternat. Med., 2014, 2014, 8 Search PubMed.
- M. Nakanishi and D. W. Rosenberg, Semin. Immunopathol., 2013, 35, 123–137 CrossRef CAS PubMed.
- D. K. Banerjee, M. V. Dhodapkar, E. Matayeva, R. M. Steinman and K. M. Dhodapkar, Blood, 2006, 108, 2655–2661 CrossRef CAS PubMed.
- J. Trinath, P. Hegde, M. Sharma, M. S. Maddur, M. Rabin, J. M. Vallat, L. Magy, K. N. Balaji, S. V. Kaveri and J. Bayry, Blood, 2013, 122, 1419–1427 CrossRef CAS PubMed.
- M. S. Maddur, J. Trinath, M. Rabin, F. Bolgert, M. Guy, J. M. Vallat, L. Magy, K. N. Balaji, S. V. Kaveri and J. Bayry, Cell. Mol. Immunol., 2015, 12, 650–652 CrossRef CAS PubMed.
- D. D. Orhan, E. Kupeli, E. Yesilada and F. Ergun, Z. Naturforsch., C: J. Biosci., 2006, 61, 26–30 CAS.
- N. Zarkovic, K. Zarkovic, S. Grainca, D. Kissel and M. Jurin, Anticancer Drugs, 1997, 8(suppl. 1), S17–S22 CrossRef CAS PubMed.
- S. Y. Lyu and W. B. Park, Arch. Pharmacal Res., 2007, 30, 1252–1264 CrossRef CAS.
- J. P. Duong Van Huyen, S. Delignat, J. Bayry, M. D. Kazatchkine, P. Bruneval, A. Nicoletti and S. V. Kaveri, Cancer Lett., 2006, 243, 32–37 CrossRef CAS PubMed.
- R. M. Steinman and J. Banchereau, Nature, 2007, 449, 419–426 CrossRef CAS PubMed.
- M. S. Maddur, M. Sharma, P. Hegde, E. Stephen-Victor, B. Pulendran, S. V. Kaveri and J. Bayry, Nat. Commun., 2014, 5, 4092 CAS.
- S. Mac Keon, M. S. Ruiz, S. Gazzaniga and R. Wainstok, Front. Immunol., 2015, 6, 243 Search PubMed.
- M. S. Maddur and J. Bayry, Oncoimmunology, 2015, 4, e1005508 CrossRef PubMed.
- K. P. Walsh and K. H. Mills, Trends Immunol., 2013, 34, 521–530 CrossRef CAS PubMed.
- S. R. Elluru, J. P. Duong van Huyen, S. Delignat, M. D. Kazatchkine, A. Friboulet, S. V. Kaveri and J. Bayry, BMC Cancer, 2008, 8, 161 CrossRef PubMed.
- S. Y. Lyu and W. B. Park, Arch. Pharmacal Res., 2011, 34, 1735–1749 CrossRef CAS PubMed.
- R. Harikrishnan, C. Balasundaram and M. S. Heo, Vet. Parasitol., 2011, 183, 146–151 CrossRef CAS PubMed.
- H. Zwierzina, L. Bergmann, H. Fiebig, S. Aamdal, P. Schoffski, K. Witthohn and H. Lentzen, Eur. J. Cancer, 2011, 47, 1450–1457 CrossRef CAS PubMed.
- M. Schink, Anticancer Drugs, 1997, 8(suppl. 1), S47–S51 CrossRef CAS PubMed.
- S. Elluru, J. P. Duong Van Huyen, S. Delignat, F. Prost, J. Bayry, M. D. Kazatchkine and S. V. Kaveri, Arzneimittelforschung, 2006, 56, 461–466 CAS.
- A. Bussing, A. Rosenberger, C. Stumpf and M. Schietzel, Forsch Komplementarmed, 1999, 6, 196–204 CAS.
- V. P. Chernyshov, P. Heusser, L. I. Omelchenko, L. I. Chernyshova, M. A. Vodyanik, E. V. Vykhovanets, L. V. Galazyuk, T. V. Pochinok, N. V. Gaiday, M. E. Gumenyuk, G. M. Zelinsky, H. Schaefermeyer and G. Schaefermeyer, Am. J. Ther., 2000, 7, 195–203 CrossRef CAS PubMed.
- S. Fischer, A. Scheffler and D. Kabelitz, Anticancer Drugs, 1997, 8(suppl. 1), S33–S37 CrossRef CAS PubMed.
- R. Huber, M. Rostock, R. Goedl, R. Ludtke, K. Urech and R. Klein, J. Soc. Integr. Oncol., 2006, 4, 3–7 Search PubMed.
- T. Hajto, K. Hostanska, K. Frei, C. Rordorf and H. J. Gabius, Cancer Res., 1990, 50, 3322–3326 CAS.
- S. Y. Lyu and W. B. Park, J. Biochem. Mol. Biol., 2006, 39, 662–670 CAS.
- T. Hajto, K. Hostanska, J. Fischer and R. Saller, Anticancer Drugs, 1997, 8(suppl. 1), S43–S46 CrossRef CAS PubMed.
- K. Hostanska, T. Hajto, G. C. Spagnoli, J. Fischer, H. Lentzen and R. Herrmann, Nat. Immun., 1995, 14, 295–304 CAS.
- I. Fidan, S. Ozkan, I. Gurbuz, E. Yesilyurt, B. Erdal, S. Yolbakan and T. Imir, Immunopharmacol. Immunotoxicol., 2008, 30, 519–528 CrossRef CAS PubMed.
- A. Bussing, K. Suzart and K. Schweizer, Anticancer Drugs, 1997, 8(suppl. 1), S9–S14 CrossRef CAS PubMed.
- J. Tabiasco, F. Pont, J. J. Fournie and A. Vercellone, Eur. J. Biochem., 2002, 269, 2591–2600 CrossRef CAS PubMed.
- G. M. Stein, G. Schaller, U. Pfuller, M. Schietzel and A. Bussing, Anticancer Res., 1999, 19, 1037–1042 CAS.
- G. M. Stein and P. A. Berg, Eur. J. Med. Res., 1998, 3, 307–314 CAS.
- G. M. Stein and P. A. Berg, Arzneimittelforschung, 1996, 46, 635–639 CAS.
- T. Hajto, K. Hostanska, K. Weber, H. Zinke, J. Fischer, U. Mengs, H. Lentzen and R. Saller, Nat. Immun., 1998, 16, 34–46 CrossRef CAS.
- Z. Liu, Y. Luo, T. T. Zhou and W. Z. Zhang, Cell Proliferation, 2013, 46, 509–515 CAS.
- S. Y. Lyu, S. H. Choi and W. B. Park, Arch. Pharmacal Res., 2002, 25, 93–101 CrossRef CAS.
- J. P. Duong Van Huyen, V. Sooryanarayana, S. Delignat, M. F. Bloch, M. D. Kazatchkine and S. V. Kaveri, Chemotherapy, 2001, 47, 366–376 CrossRef CAS PubMed.
- G. M. Stein, U. Pfuller and M. Schietzel, Anticancer Res., 1999, 19, 2925–2928 CAS.
- T. J. Zuzak, L. Rist, J. Eggenschwiler, M. A. Grotzer and A. Viviani, Anticancer Res., 2006, 26, 3485–3492 CAS.
- M. Frantz, M. L. Jung, G. Ribereau-Gayon and R. Anton, Arzneimittelforschung, 2000, 50, 471–478 CAS.
- A. Bussing and M. Schietzel, Anticancer Res., 1999, 19, 23–28 CAS.
- J. H. Park, C. K. Hyun and H. K. Shin, Cancer Lett., 1999, 139, 207–213 CrossRef CAS PubMed.
- T. J. Yoon, Y. C. Yoo, T. B. Kang, K. Shimazaki, S. K. Song, K. H. Lee, S. H. Kim, C. H. Park, I. Azuma and J. B. Kim, Cancer Lett., 1999, 136, 33–40 CrossRef CAS PubMed.
- W. B. Park, S. Y. Lyu, J. H. Kim, S. H. Choi, H. K. Chung, S. H. Ahn, S. Y. Hong, T. J. Yoon and M. J. Choi, Cancer Biother. Radiopharm., 2001, 16, 439–447 CrossRef CAS PubMed.
- O. Podlech, P. N. Harter, M. Mittelbronn, S. Schel and U. Naumann, J. Evidence-Based Complementary Altern. Med., 2012, 2012, 15 Search PubMed.
- V. Lavastre, S. Chiasson, H. Cavalli and D. Girard, Leuk. Res., 2005, 29, 1443–1453 CrossRef CAS PubMed.
- V. Lavastre, H. Cavalli, C. Ratthe and D. Girard, Clin. Exp. Immunol., 2004, 137, 272–278 CrossRef CAS PubMed.
- V. Lavastre, M. Pelletier, R. Saller, K. Hostanska and D. Girard, J. Immunol., 2002, 168, 1419–1427 CrossRef CAS.
- M. Harmsma, M. Gromme, M. Ummelen, W. Dignef, K. J. Tusenius and F. C. Ramaekers, Int. J. Oncol., 2004, 25, 1521–1529 CAS.
- M. J. Jung, Y. C. Yoo, K. B. Lee, J. B. Kim and K. S. Song, Arch. Pharmacal Res., 2004, 27, 840–844 CrossRef CAS.
- W. H. Kim, W. B. Park, B. Gao and M. H. Jung, Mol. Pharmacol., 2004, 66, 1383–1396 CrossRef CAS PubMed.
- R. Park, M. S. Kim, H. S. So, B. H. Jung, S. R. Moon, S. Y. Chung, C. B. Ko, B. R. Kim and H. T. Chung, Biochem. Pharmacol., 2000, 60, 1685–1691 CrossRef CAS PubMed.
- H. O. Pae, W. G. Seo, M. Shin, H. S. Lee, S. B. Kim and H. T. Chung, Immunopharmacol. Immunotoxicol., 2000, 22, 279–295 CrossRef CAS PubMed.
- A. Bussing, M. Wagner, B. Wagner, G. M. Stein, M. Schietzel, G. Schaller and U. Pfuller, Cancer Lett., 1999, 139, 79–88 CrossRef CAS PubMed.
- E. Onay-Ucar, O. Erol, B. Kandemir, E. Mertoglu, A. Karagoz and N. Arda, Evid. Based Complement Alternat. Med., 2012, 2012, 958740 Search PubMed.
- B. K. Kim, M. J. Choi, K. Y. Park and E. J. Cho, Biol. Pharm. Bull., 2010, 33, 1152–1158 Search PubMed.
- E. Onay-Ucar, A. Karagoz and N. Arda, Fitoterapia, 2006, 77, 556–560 CrossRef PubMed.
- M. Namuslu, H. Kocaoglu, H. T. Celik, A. Avci, E. Devrim, Y. Genc, E. Gocmen, I. B. Erguder and I. Durak, Bratisl. Lek. Listy, 2014, 115, 367–371 CAS.
- M. Estko, S. Baumgartner, K. Urech, M. Kunz, U. Regueiro, P. Heusser and U. Weissenstein, BMC Complementary Altern. Med., 2015, 15, 130 CrossRef PubMed.
- R. L. Stan, A. C. Hangan, L. Dican, B. Sevastre, D. Hanganu, C. Catoi, O. Sarpataki and C. M. Ionescu, Acta Biol. Hung., 2013, 64, 279–288 CrossRef PubMed.
- E. O. Ucar, N. Arda and A. Aitken, GMR, Genet. Mol. Res., 2012, 11, 2801–2813 CrossRef CAS PubMed.
- I. Siegle, P. Fritz, M. McClellan, S. Gutzeit and T. E. Murdter, Anticancer Res., 2001, 21, 2687–2691 CAS.
- M. S. Kim, H. S. So, K. M. Lee, J. S. Park, J. H. Lee, S. K. Moon, D. G. Ryu, S. Y. Chung, B. H. Jung, Y. K. Kim, G. Moon and R. Park, Gen. Pharmacol., 2000, 34, 349–355 CrossRef CAS PubMed.
- M. S. Kim, J. Lee, H. S. So, K. M. Lee, B. H. Jung, S. Y. Chung, S. R. Moon, N. S. Kim, C. B. Ko, H. J. Kim, Y. K. Kim and R. Park, Immunopharmacol. Immunotoxicol., 2001, 23, 55–66 CrossRef CAS PubMed.
- A. Bussing, A. S. Multani, S. Pathak, U. Pfuller and M. Schietzel, Cancer Lett., 1998, 130, 57–68 CrossRef CAS PubMed.
- V. C. George, D. Kumar, P. Suresh and R. A. Kumar, Asian Pacific Journal of Cancer Prevention: APJCP, 2012, 13, 2015–2020 CrossRef PubMed.
- C. I. Delebinski, S. Jaeger, K. Kemnitz-Hassanin, G. Henze, H. N. Lode and G. J. Seifert, Cell Proliferation, 2012, 45, 176–187 CrossRef CAS PubMed.
- M. D. Mossalayi, A. Alkharrat and D. Malvy, Arzneimittelforschung, 2006, 56, 457–460 CAS.
- G. Kelter and H. H. Fiebig, Arzneimittelforschung, 2006, 56, 435–440 CAS.
- G. Maier and H. H. Fiebig, Anticancer Drugs, 2002, 13, 373–379 CrossRef CAS PubMed.
- K. Urech, A. Buessing, G. Thalmann, H. Schaefermeyer and P. Heusser, Anticancer Res., 2006, 26, 3049–3055 Search PubMed.
- J. L. Kong, X. B. Du, C. X. Fan, J. F. Xu and X. J. Zheng, Yaoxue Xuebao, 2004, 39, 813–817 CAS.
- S. H. Choi, S. Y. Lyu and W. B. Park, Arch. Pharmacal Res., 2004, 27, 68–76 CrossRef CAS.
- F. Y. Zeng, A. Benguria, S. Kafert, S. Andre, H. J. Gabius and A. Villalobo, Mol. Cell. Biochem., 1995, 142, 117–124 CrossRef CAS PubMed.
- T. J. Yoon, Y. C. Yoo, T. B. Kang, S. K. Song, K. B. Lee, E. Her, K. S. Song and J. B. Kim, Arch. Pharmacal Res., 2003, 26, 861–867 CrossRef CAS.
- T. J. Yoon, Y. C. Yoo, T. B. Kang, Y. J. Baek, C. S. Huh, S. K. Song, K. H. Lee, I. Azuma and J. B. Kim, Int. J. Immunopharmacol., 1998, 20, 163–172 CrossRef CAS PubMed.
- G. Kuttan, L. G. Menon, S. Antony and R. Kuttan, Anticancer Drugs, 1997, 8(suppl. 1), S15–S16 CrossRef CAS PubMed.
- E. Kunze, H. Schulz and H. J. Gabius, J. Cancer Res. Clin. Oncol., 1998, 124, 73–87 CrossRef CAS PubMed.
- K. Weber, U. Mengs, T. Schwarz, T. Hajto, K. Hostanska, T. R. Allen, R. Weyhenmeyer and H. Lentzen, Arzneimittelforschung, 1998, 48, 497–502 CAS.
- T. Srdic-Rajic, N. Tisma-Miletic, M. Cavic, K. Kanjer, K. Savikin, D. Galun, A. Konic-Ristic and T. Zoranovic, Phytother. Res., 2015 DOI:10.1002/ptr.5554.
- K. W. Kim, S. H. Yang and J. B. Kim, Evid. Based Complement Alternat. Med., 2014, 2014, 703624 Search PubMed.
- R. Mikeska, R. Wacker, R. Arni, T. P. Singh, A. Mikhailov, A. Gabdoulkhakov, W. Voelter and C. Betzel, Acta Crystallogr., Sect. F: Struct. Biol. Cryst. Commun., 2005, 61, 17–25 CrossRef CAS PubMed.
- S. Önal, S. Timur, B. Okutucu and F. Zihnioğlu, Prep. Biochem. Biotechnol., 2005, 35, 29–36 CrossRef PubMed.
- A. M. Gray and P. R. Flatt, J. Endocrinol., 1999, 160, 409–414 CrossRef CAS PubMed.
- M. Radenkovic, V. Ivetic, M. Popovic, S. Brankovic and L. Gvozdenovic, Clin. Exp. Hypertens., 2009, 31, 11–19 CrossRef CAS PubMed.
- M. Sengul, H. Yildiz, N. Gungor, B. Cetin, Z. Eser and S. Ercisli, Pak. J. Pharm. Sci., 2009, 22, 102–106 CAS.
- A. Karagoz, E. Onay, N. Arda and A. Kuru, Phytother. Res., 2003, 17, 560–562 CrossRef PubMed.
- K. J. Tusenius, J. M. Spoek and C. W. Kramers, Compl. Ther. Med., 2001, 9, 12–16 CrossRef CAS PubMed.
- M. Stoss and R. W. Gorter, Nat. Immun., 1998, 16, 157–164 CrossRef CAS PubMed.
- C. E. Hong and S. Y. Lyu, Phytother. Res., 2012, 26, 787–790 CrossRef CAS PubMed.
- T. von Schoen-Angerer, R. Madeleyn, G. Kienle, H. Kiene and J. Vagedes, J. Child Neurol., 2015, 30, 1048–1052 CrossRef PubMed.
- R. Kuonen, U. Weissenstein, K. Urech, M. Kunz, K. Hostanska, M. Estko, P. Heusser and S. Baumgartner, Evid. Based Complement Alternat. Med., 2013, 2013, 718105 CAS.
- S. H. Lee, H. S. An, Y. W. Jung, E. J. Lee, H. Y. Lee, E. S. Choi, S. W. An, H. Son, S. J. Lee, J. B. Kim and K. J. Min, Biogerontology, 2014, 15, 153–164 CrossRef PubMed.
- H.-Y. Jung, Y.-H. Kim, I.-B. Kim, J. S. Jeong, J.-H. Lee, M.-S. Do, S.-P. Jung, K.-S. Kim, K.-T. Kim and J.-B. Kim, Evid. Based Complement Alternat. Med., 2013, 2013, 9 Search PubMed.
- H. Y. Jung, A. N. Lee, T. J. Song, H. S. An, Y. H. Kim, K. D. Kim, I. B. Kim, K. S. Kim, B. S. Han, C. H. Kim and J. B. Kim, J. Med. Food, 2012, 15, 621–628 CrossRef PubMed.
- Y. M. Lee, Y. S. Kim, Y. Lee, J. Kim, H. Sun, J. H. Kim and J. S. Kim, Phytother. Res., 2012, 26, 778–782 CrossRef CAS PubMed.
- G. Avcı, E. Kupeli, A. Eryavuz, E. Yesilada and I. Kucukkurt, J. Ethnopharmacol., 2006, 107, 418–423 CrossRef PubMed.
- F. A. Tenorio, L. del Valle, A. Gonzalez and G. Pastelin, Fitoterapia, 2005, 76, 204–209 CrossRef CAS PubMed.
- A. B. Samal, A. V. Timoshenko, E. N. Loiko, H. Kaltner and H. J. Gabius, Biochemistry, 1998, 63, 516–522 CAS.
- G. S. Kienle and H. Kiene, Eur. J. Med. Res., 2007, 12, 103–119 CAS.
- J. Melzer, F. Iten, K. Hostanska and R. Saller, Forsch Komplementmed, 2009, 16, 217–226 Search PubMed.
- G. S. Kienle, F. Berrino, A. Bussing, E. Portalupi, S. Rosenzweig and H. Kiene, Eur. J. Med. Res., 2003, 8, 109–119 CAS.
- T. Ostermann, C. Raak and A. Bussing, BMC Cancer, 2009, 9, 451 CrossRef PubMed.
- G. S. Kienle and H. Kiene, Integr. Cancer Ther., 2010, 9, 142–157 CrossRef PubMed.
- E. Ernst, K. Schmidt and M. K. Steuer-Vogt, Int. J. Cancer, 2003, 107, 262–267 CrossRef CAS PubMed.
- M. A. Horneber, G. Bueschel, R. Huber, K. Linde and M. Rostock, Cochrane Database of Systematic Reviews, 2008, CD003297 CAS.
- I. Gerhard, U. Abel, A. Loewe-Mesch, S. Huppmann and J. J. Kuehn, Forsch Komplementarmed Klass Naturheilkd, 2004, 11, 150–157 CAS.
- W. Troger, D. Galun, M. Reif, A. Schumann, N. Stankovic and M. Milicevic, Eur. J. Cancer, 2013, 49, 3788–3797 CrossRef CAS PubMed.
- W. Troger, D. Galun, M. Reif, A. Schumann, N. Stankovic and M. Milicevic, Deutsches Ärzteblatt International, 2014, 111, 493–502 Search PubMed.
- W. Troger, S. Jezdic, Z. Zdrale, N. Tisma, H. J. Hamre and M. Matijasevic, Breast Cancer, 2009, 3, 35–45 CAS.
- W. Troger, Z. Zdrale, N. Stankovic and M. Matijasevic, Breast Cancer, 2012, 6, 173–180 Search PubMed.
- A. Longhi, M. Reif, E. Mariani and S. Ferrari, Evid. Based Complement Alternat. Med., 2014, 2014, 210198 Search PubMed.
- G. Bar-Sela, M. Wollner, L. Hammer, A. Agbarya, E. Dudnik and N. Haim, Eur. J. Cancer, 2013, 49, 1058–1064 CrossRef CAS PubMed.
- B. K. Piao, Y. X. Wang, G. R. Xie, U. Mansmann, H. Matthes, J. Beuth and H. S. Lin, Anticancer Res., 2004, 24, 303–309 CAS.
- P. J. Mansky, D. B. Wallerstedt, T. S. Sannes, J. Stagl, L. L. Johnson, M. R. Blackman, J. L. Grem, S. M. Swain and B. P. Monahan, Evid. Based Complement Alternat. Med., 2013, 2013, 11 Search PubMed.
- P. R. Bock, J. Hanisch, H. Matthes and K. S. Zanker, Inflammation Allergy: Drug Targets, 2014, 13, 105–111 CrossRef CAS PubMed.
- M. L. Steele, J. Axtner, A. Happe, M. Kroz, H. Matthes and F. Schad, Evid. Based Complement Alternat. Med., 2014, 2014, 724258 Search PubMed.
- J. Beuth, H. J. Gabius, M. K. Steuer, J. Geisel, M. Steuer, H. L. Ko and G. Pulverer, Med. Klin., 1993, 88, 287–290 CAS.
- A. Bussing, G. M. Stein, M. Wagner, B. Wagner, G. Schaller, U. Pfuller and M. Schietzel, Eur. J. Biochem., 1999, 262, 79–87 CrossRef CAS PubMed.
- G. Stein, MISTLETOE: The Genus Viscum, Harwood academic publishers, Germany, 2000 Search PubMed.
- M. Sauviat, Toxicon, 1990, 28, 83–92 CrossRef CAS PubMed.
- J. Maldacker, Arzneimittelforschung, 2006, 56, 497–507 CAS.
- K. Winterfeld, Pharm. Ztg., 1952, 88, 573–574 Search PubMed.
- A. H. Hall, D. G. Spoerke and B. H. Rumack, Ann. Emerg. Med., 1986, 15, 1320–1323 CrossRef CAS PubMed.
- J. Harvey and D. G. Colin-Jones, Br. Med. J., 1981, 282, 186–187 CrossRef CAS PubMed.
- G. Weeks and J. S. Proper, Aust. J. Hosp. Pharm., 1989, 19, 155–157 Search PubMed.
- J. A. L. Nienhaus, Elemente der Naturw, 1970, 13, 45–54 Search PubMed.
- L. E. Rentea and R. Hunter, Lab. Invest., 1981, 44, 43–48 Search PubMed.
- T. Hajto, Oncology, 1986, 43, 51–65 CrossRef PubMed.
- P. Luther, G. Uhlenbruck, H. Reutgen, R. Samtleben, I. Sehrt and G. Ribereau-Gayon, Z. Erkr. Atmungsorgane, 1986, 166, 247–256 CAS.
- U. Mengs, in Effects of antinutrients on the nutritional value of legume diets, ed. S. Bardocz, U. Pfüller and A. Pusztai, Office for Official Publications of the European Communites, 1998, vol. 5, pp. 77–80, COST 98 Search PubMed.
- H. Franz, A. Kindt, P. Ziska, H. Bielka, R. Benndorf and L. Venker, Acta Biol. Med. Ger., 1982, 41, K9–K16 CAS.
- L. R. Stirpe, L. J. Onyon, P. Ziska and H. Franz, Biochem. J., 1980, 190, 843–848 CrossRef PubMed.
- H. Franz, Adv. Lectin Res., 1991, 4, 33–50 CAS.
- J. P. D. Van Huyen, S. Delignat, J. Bayry, M. D. Kazatchkine, P. Bruneval, A. Nicoletti and S. V. Kaveri, Cancer Lett., 2006, 243, 32–37 CrossRef PubMed.
- W. B. Park, S. Y. Lyu, J. H. Kim, S. H. Choi, H. K. Chung, S. H. Ahn, S. Y. Hong, T. J. Yoon and M. J. Choi, Cancer Biother. Radiopharm., 2001, 16, 439–447 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |