Durable and modified foam for cleanup of oil contamination and separation of oil–water mixtures†
Abstract
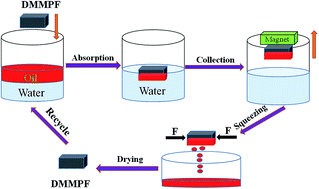
With the continuous exploitation and utilization of oil resources, oil pollution has become increasingly serious, which not only causes economic loss, but also damages the living environment of human beings. Herein, oleic acid coated Fe3O4 particles and PS microspheres were introduced to the surface of pure foam through an inexpensive two-step immersion method; we obtained a durable and modified magnetic polystyrene foam (DMMPF) with high efficiency and selectivity. The as-obtained DMMPF exhibited superhydrophobicity, superoleophilicity, and fast magnetic response via the surface chemical modification. In addition, the as-prepared DMMPF could be used for continuous separation of various oils and organic solvents from the water surface. The absorption intake capacity of DMMPF was 40.1 times its own weight and the absorbed oils/organic solvents could be collected by simple mechanical extrusion. Furthermore, the as-prepared DMMPF retained an excellent absorption capacity and a large water contact angle after 60 separation cycles. It is recognized that DMMPF could be suitable for large-scale oil spill processing.

 Please wait while we load your content...
Please wait while we load your content...