One-pot synthesis of La-doped SnO2 layered nanoarrays with an enhanced gas-sensing performance toward acetone
Abstract
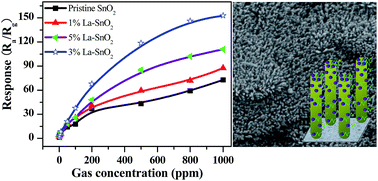
Lanthanum doped SnO2 well-oriented layered nanorod arrays were synthesized by a substrate-free hydrothermal route of using sodium stannate and sodium hydroxide at 210 °C. The morphology and phase structure of the La-doped SnO2 nanoarrays were investigated by X-ray powder diffraction spectroscopy, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy and the BET method. The results showed that the La-doped SnO2 layered nanorod array demonstrated a unique nanostructure combined together with double layers of nanorod arrays and could be indexed to a tetragonal structure. The gas sensing performance of La-doped SnO2 nanoarrays indicated that La doping could enhance the sensing response to acetone. The 3.0 at% La-doped level of the SnO2 sensor not only showed good selectivity and excellent stability, but also exhibited a rapid response and recovery compared to the pristine and other La-doped levels of SnO2 nanoarrays. The gas sensing mechanism of the La-doped SnO2 layered nanoarray was discussed. The La-doped SnO2 sensors are considered to be promising candidates for applications in detecting acetone.

 Please wait while we load your content...
Please wait while we load your content...