Surface clean gold nanoflower obtained by complete removal of capping agents: an active catalyst for alcohol oxidation†
Abstract
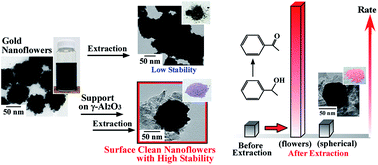
Shape-controlled metal nanocrystals, such as nanowires and nanoflowers (NFs), are attractive due to their potential novel catalytic properties. However, catalytic activity is limited due to capping agents adsorbed on the metal surface, which are necessary for the preparation and dispersion of shape-controlled nanocrystals in solution. Recently, surface clean shape-controlled nanocrystals, which are obtained by removal of almost all of the capping agent, are very interested to bring out the intrinsically novel catalytic property. In this study, to prepare surface clean shape-controlled nanocrystals, firstly, Au NFs obtained by surfactant-free synthesis were supported on γ-Al2O3 to increase dispersibility and morphological stability, because unsupported Au NFs formed aggregates by removal of the capping agent. Then, we removed the capping agent from the surface of Au NFs by a calcination procedure and a water extraction procedure, in order to improve their catalytic activity. The water extraction procedure succeeded in removing the capping agent from Au NFs/γ-Al2O3 without morphological change and in providing surface clean Au NFs/γ-Al2O3, while the calcination procedure changed the morphology from NFs to spherical Au nanoparticles (Au NPs). Surface clean Au NFs/γ-Al2O3 was used for the aerobic oxidation of 1-phenylethyl alcohol, and the extraction procedure resulted in an eleven-fold increase in catalytic activity. Furthermore, the formation rate of acetophenone on surface clean Au NFs/γ-Al2O3 was ten-fold higher than that on surface clean Au NPs/γ-Al2O3 having almost the same diameter, indicating the catalytic superiority of surface clean Au NFs.

 Please wait while we load your content...
Please wait while we load your content...