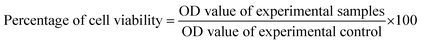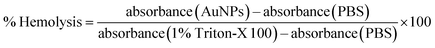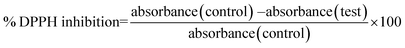Longan fruit juice mediated synthesis of uniformly dispersed spherical AuNPs: cytotoxicity against human breast cancer cell line MCF-7, antioxidant and fluorescent properties
Arif Ullah Khan*a,
Qipeng Yuan*a,
Yun Weia,
Shahab Ullah Khanc,
Kamran Tahira,
Zia Ul Haq Khanb,
Aftab Ahmada,
Farman Alid,
Shafqat Alia and
Sadia Nazira
aState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing, PR China. E-mail: yuanqp@mail.buct.edu.cn; khanbuct@gmail.com; Fax: +86-10-6443761; Tel: +86-10-64431557
bDepartment of Chemistry, University of Science and Technology Bannu, KP, Pakistan
cDepartment of Physics, University of Peshawar, 25000, Pakistan
dDepartment of Chemistry, Shaheed Benazir Bhutto University, Sheringal, Dir 18000, KP, Pakistan
First published on 22nd February 2016
Abstract
The development of a biologically glorious experimental process for the synthesis of nanoparticles is an important emerging branch of nanotechnology. Gold nanoparticles (AuNPs) can be easily synthesized, functionalized and are biocompatible. In the present work AuNPs were synthesized by an eco-friendly, fast, one-pot and green synthetic route using Longan fruit juice as a reducing, capping and stabilizing agent. The AuNPs showed surface plasmon resonance at around 555 nm. The spherical shape, fine dispersion, average size (25 nm), crystalline structure and elemental composition were characterized by high resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD) and energy-dispersive X-ray spectroscopy (EDS). The capping and stabilizing molecules were characterized by Fourier transform infrared spectroscopy (FTIR). The AuNPs were found to be unique with respect to mono-structural morphology and greater yield as compared to other bio-synthesized AuNPs. Furthermore, the cytotoxicity of AuNPs proved to be potent agent against human breast cancer cell line MCF-7. AuNPs exhibited significant antioxidant activity and fluorescence emission. These biomaterials can be used for broad biomedical applications.
1. Introduction
Nanomaterials have a great role in improving human life and the environment.1 The physical properties of bulk materials remain constant regardless of their size but at the nano-scale often this is not true. There are many factors for this, including that nanoparticles have a very high surface to volume ratio. Nanoparticles bridge the gap between bulk materials at the atomic and molecular level, so they are of great scientific interest.2 Many researchers are interested in synthesizing nanomaterials with complex morphology and controlling their size and shape which is essential in tuning their properties and uses.3,4 In the last few years, AuNPs have received attention in research because of their unique optical, electrical and photothermal properties.5 Moreover, they are highly stable to oxidation.6 Physical and chemical properties of AuNPs depend upon phase and morphology variations.7 AuNPs have advantages over other metal nanoparticles due to their biocompatibility and non-cytotoxicity. Nanoparticles are of nanometer size. Gold is used internally in humans for the last 50 years due to its chemical inertness. The size of AuNPs can be controlled during their synthesis and particle functionalization with different groups. AuNPs accumulate in tumor cells and show optical scattering. Therefore, these can act as a probe for microscopy studies of cancer cells. These are also used in chemotherapy and diagnosis of a cancer cell.8,9 AuNPs are capable of delivering large biomolecules, not restricting themselves as carriers of only small molecular drugs.10Many protocols are in practice to synthesize metallic nanoparticles such as electrochemical, sonochemical and microwave assisted processes, but nearly all of these processes suffer from utilization of high energy, hazardous chemicals and difficulties in purification.11 Development of improved and environmentally benign methods is necessary for the design of greener nanomaterials with high reproducibility and purity. In terms of a greener production, one would like to avoid the use of hazardous materials and minimize the production of hazardous byproducts.12 Development of green chemistry methods for efficient synthesis of metal nanoparticles by using plant extracts13–19 is an interesting area of nanotechnology, which has economic and eco-friendly benefits over chemical and physical methods of synthesis.20
The biomolecules present in plants include various water soluble metabolites (e.g. alkaloids, phenolic compounds, and terpenoids) and co-enzymes can be used to reduce metal ions to nanoparticles in a single-step green synthesis process. The reduction of metal ions in the presence of plants metabolites could be conducted readily at room temperature and pressure and could be easily expanded to the commercial scale. The organic compounds of plants may act both as reducing and stabilizing agents in the synthesis of nanoparticles.21,22
Fruit of Euphoria longana Lam. (Longan) are consumed throughout Asia and are a major crop in Thailand. The major components of fruit of Euphoria longana Lam. (Longan) are gallic acid, corilagin (an ellagitannin) and ellagic acid.23 These naturally occurring compounds are polyphenols, so can be proven to be the best reducing, capping and stabilizing agents of AuNPs.
To the best of our knowledge it is the first report about the novel and green synthesis of spherical AuNPs using Longan fruit juice as a reducing, capping and stabilizing agent without the use of any external chemical. AuNPs are evaluated for their anticancer response against the breast cancer cell line MCF 7, antioxidant activity and fluorescence emission properties.
2. Materials and methods
2.1. Materials
Longan fruit was purchased from a local market. Chloroauric acid (HAuCl4·3H2O), dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and Dulbecco’s Modified Eagle Medium (DMEM) were purchased from Beijing chemical works, China. All solutions were made in double distilled water and DMEM. All apparatuses were washed with aqua regia and rewashed with double distilled water.2.2. Juice extraction
Longan fresh fruits were purchased from a local market in Beijing. The Longan fruit were hand peeled and the flesh juice was removed using a juice extractor. The juice was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 minutes at 4 °C to remove the solid material. The supernatant was used as a reducing, capping and stabilizing agent of AuNPs.
000 rpm for 15 minutes at 4 °C to remove the solid material. The supernatant was used as a reducing, capping and stabilizing agent of AuNPs.
2.3. AuNPs synthesis
20 mL of Longan fruit juice was mixed with 50 mL of 6 × 10−3 M aqueous solution of HAuCl4 in a 100 mL beaker to synthesize AuNPs. The reaction mixture was stirred at 300 rpm using a magnetic stirrer at 30 °C and the formation of AuNPs was observed by visual observation of a color change from whitish to dark brown. The progress of AuNP synthesis was regularly observed by UV-Vis spectroscopy and it was found that the reaction was completed after 30 min because there was no further change in the surface plasmon resonance (SPR) peak intensity at 555 nm even for 60 min. AuNPs were separated from the obtained suspension by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The AuNP pellet was washed twice with double distilled water and re-centrifuged at the same conditions to remove the uncoated and suspended juice material. Then the AuNPs were freeze dried using a VirTis freeze mobile 6ES freeze drier and kept for further characterization and applications.
000 rpm for 10 min. The AuNP pellet was washed twice with double distilled water and re-centrifuged at the same conditions to remove the uncoated and suspended juice material. Then the AuNPs were freeze dried using a VirTis freeze mobile 6ES freeze drier and kept for further characterization and applications.
2.4. Characterization
The biosynthesis of AuNPs was monitored frequently by scanning the aliquot sample in the wavelength range of 400–700 nm and recording the surface plasmon resonance (SPR) peak of the AuNPs using a UV-Vis 2450 spectrophotometer (Shimadzu). The XRD measurements were examined using a Rigaku Miniflex X-ray diffractometer to determine the crystalline structure of AuNPs. A Hitachi EDS elemental microanalysis system and JEOL 3010 high resolution transmission electron microscope were used to determine the elemental composition, morphology and size of the AuNPs. A infrared (IR) spectrum was obtained using the KBr pellet technique on an ABB MB 3000 spectrophotometer to determine the stabilizing and capping molecules of AuNPs.Thermogravimetric analysis (TGA) of biosynthesized Au NPs was conducted on a NETZSCH STAR 449C system (33–750 °C) with a heating rate of 10 °C min−1 under nitrogen atmosphere.
2.5. Cytotoxicity
MCF-7 cell lines were taken from the College of Life Science and Technology, Beijing University of Chemical Technology, Beijing, China, where they were stored in liquid nitrogen. The cell lines were grown in 75 cm2 flasks using DMEM supplemented with 2 mM L-glutamine, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin and 10% FBS. The cells were seeded into 96 well plates (5 × 103 cells per well) and incubated for 24 h at 37 °C in 5% CO2 atmosphere. MCF-7 cells were treated with different concentrations of AuNPs (6.25, 12.5, 25, 50 and 100 μg mL−1). The AuNPs were dissolved in DMEM. DMEM without AuNPs was used as the experimental control. The plates were incubated for 24 h in order to perform a cell viability assay, the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Then 20 μL of MTT (5 mg mL−1) was added to each well and incubated for 4 h. Purple color formazone crystals formed and were dissolved in 100 μL of dimethyl sulfoxide (DMSO). The optical density was monitored at 570 nm in a multi well ELISA plate reader. The optical density value was used to calculate the percentage of viability according to the following formula:2.6. Hemolytic activity assay
The in vitro hemolytic property of AuNPs was assessed by measuring hemoglobin release from red blood cells (RBCs) following treatment with AuNPs. Blood was collected from male Wistar albino mice in sterile lithium heparin vacutainers. The whole blood was diluted with phosphate buffered saline (PBS) and was centrifuged at 1500 rpm for 10 minutes. The supernatant and buffy coat was carefully removed and the pellet (RBCs) was washed three times with PBS at pH 7.4 until the supernatant was clear. The pellet was resuspended in 5 mL of PBS and the RBCs were counted using a haemocytometer. AuNPs were diluted to the required concentration in PBS and different concentrations (12.5, 25, 50, 75 and 100 μg mL−1) were put into separate tubes. Approximately, 70 million cells per mL were transferred to each tube for hemolysis. RBCs in PBS and 1% Triton-X 100 solution was taken as the negative and positive control respectively. These reaction mixtures were incubated in a shaker incubator at 37 °C for 1 h with gentle shaking (180 shakes per min). Then the tubes were centrifuged at 1500 rpm for 10 minutes to remove the RBCs as well as the colloidal AuNPs. The supernatant was recentrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to make sure that the supernatant is clear from AuNPs. Then the clear supernatant was monitored at 540 nm against the blank. The percentage of hemolytic activity was determined as previously reported:24
000 rpm for 5 min to make sure that the supernatant is clear from AuNPs. Then the clear supernatant was monitored at 540 nm against the blank. The percentage of hemolytic activity was determined as previously reported:242.7. DPPH free radical scavenging assay
DPPH free radical scavenging assay for AuNPs was performed as previously described,25 with a little modification. 0.5 mL of 1 mM DPPH was separately mixed with different concentrations (0.031, 0.062, 0.125, 0.250, 0.500 and 1 mg mL−1) of AuNPs dissolved in methanol and incubated in the dark for 30 minutes. After incubation the samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to remove the suspended AuNPs and the absorbance of the clear supernatants was determined using a UV-1100 spectrophotometer (MAPADA instruments) at 517 nm against methanol as a blank. Vit.C was used as a standard and DPPH methanol reagent without sample was used as a control. The percentage of inhibition was calculated by the following formula:
000 rpm for 5 min to remove the suspended AuNPs and the absorbance of the clear supernatants was determined using a UV-1100 spectrophotometer (MAPADA instruments) at 517 nm against methanol as a blank. Vit.C was used as a standard and DPPH methanol reagent without sample was used as a control. The percentage of inhibition was calculated by the following formula:2.8. Fluorescence emission
The instrument used in this experiment was a Cary Eclipse fluorescence spectrophotometer which was controlled by Cary Eclipse software on a Pentium PC. The instrument consists of two Czerny–Turner monochromators (excitation and emission), a xenon light source, a range of fixed width selectable slits, selectable filters, attenuators and two photomultiplier tubes as detectors. The light containing the fluorescent signal from the sample is viewed at 90° with respect to the incident beam. The emitted light from the cell is directed into the emission monochromator, consisting of a motorized grating. The detector (photomultiplier tube) measures the spectral light intensity. The fluorescence emission spectrum was measured under excitation at 555 nm.3. Results and discussion
3.1. UV-visible spectroscopy
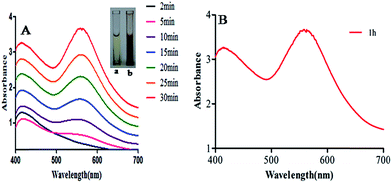
The mixture of HAuCl4·3H2O and Longan fruit juice showed a color change from whitish to dark brown within 30 minutes. This color appears due to localized surface plasmon resonance (LSPR) in AuNPs. The characteristic LSPR peak was not observed at the initial stage but after 5 minutes the free electrons of AuNPs give rise to the LSPR peak.26UV-Vis spectra of the reaction medium was recorded as a function of time (Fig. 1) where the inset (a, b) is the color change during the completion of AuNP synthesis. Fig. 1A shows that the intensity and sharpness of the LSPR peak increases with increasing contact time and the reaction terminates in 30 minutes as it does not show any further change in absorbance and LSPR peak intensity even for 60 min (Fig. 1B). It showed the maximum absorbance at 555 nm, which depends upon the size, shape, dispersion and the surrounding media of AuNPs. LSPR gives rise to a peak which is well-documented for various Au metallic nanoparticles ranging from 2 nm to 100 nm.27
3.2. HRTEM and EDS analysis
The size and morphology of AuNPs were characterized by HRTEM. The HRTEM image (Fig. 2A) shows that the AuNPs are spherical and uniformly dispersed. There is no aggregation among AuNPs which shows the high reducing, capping and stabilizing ability of phytochemicals found in Longan fruit juice. The phytochemicals induce excellent capping of AuNPs and cause their uniform distribution and inhibit aggregation. The white lining around the AuNPs show the presence of capping and stabilizing agents (phytochemicals) found in Longan fruit juice.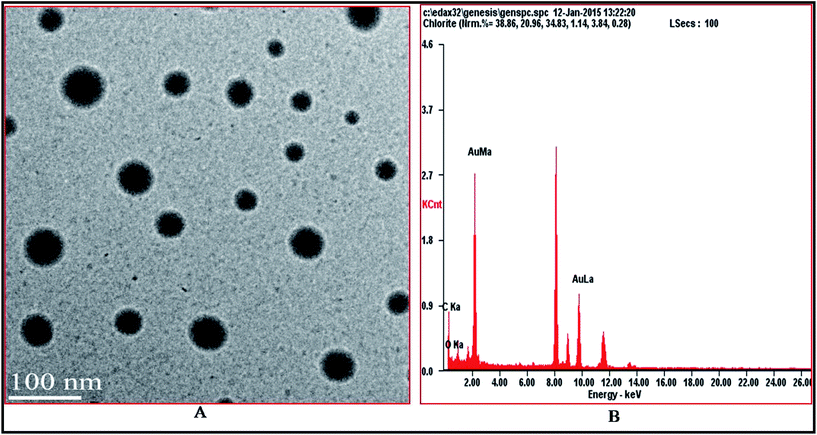 | ||
| Fig. 2 HRTEM image (A) showing spherical shape and uniform distribution of AuNPs and (B) EDS profile showing elemental composition of AuNPs. | ||
The average particle size measured from the HRTEM image is 25 nm. The smaller, spherical particle size and high dispersity of AuNPs leads to more active sites and a large surface area.
The EDS profile of AuNPs showed elemental composition and strong signals for gold atoms as shown in Fig. 2B. EDS analysis mostly showed strong signal energy peaks for Au atoms in the range of 2 keV,17 which is a typical signal for the absorption of metallic AuNPs due to LSPR.28
3.3. XRD and FTIR analysis
X-ray diffraction analysis at 20–80° confirmed the crystal structure of AuNPs (Fig. 3A). Different numbers of Braggs’ reflection with 2 theta values of 38.12°, 44.12°, 63.34° and 79° represent the (111), (200), (220) and (311) set of lattice planes respectively, which may be indexed to the face centered cubic structure of AuNPs, which are in agreement with JCPDS file number 00-004-0783. The intensity of the peak corresponding to (111) is higher than that of the other lattice planes suggesting that (111) is the main orientation. The average size of the particle is calculated using Debye–Scherrer eqn (1) considering the width of the (111) peak and was found to be 27 nm which agrees with the particle size obtained from the HRTEM image.
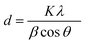 | (1) |
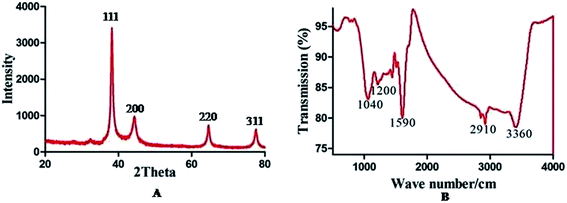 | ||
| Fig. 3 XRD pattern (A) confirming the crystalline nature of AuNPs and FTIR (B) indicating various major functional groups responsible for capping and stabilization of AuNPs. | ||
Bio-molecules responsible for capping and stabilization of AuNPs were confirmed by viewing the FTIR spectrum (Fig. 3B). FTIR spectrum shows absorption peaks at 3360 cm−1 and 1590 cm−1 corresponding to O–H stretching vibration and aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration respectively.29 The strong and broad peak observed at 1040 cm−1, may be due to the presence of C–N stretching vibrations of aliphatic amines present in plant proteins. This evidence reveals that the amine linkage in protein molecules can possibly be involved in the reduction of Au3+ ions and stabilization of gold nano conjugates in aqueous medium.30 The peak at 1386 cm−1 was attributed to –C–O– (ether) stretching vibration.31 While peaks at 2910 and 1200 cm−1 are for C–H and C–N stretches respectively. The FTIR spectrum also provided an idea about bio-molecules bearing different functionalities which are present in the underlying system.
C stretching vibration respectively.29 The strong and broad peak observed at 1040 cm−1, may be due to the presence of C–N stretching vibrations of aliphatic amines present in plant proteins. This evidence reveals that the amine linkage in protein molecules can possibly be involved in the reduction of Au3+ ions and stabilization of gold nano conjugates in aqueous medium.30 The peak at 1386 cm−1 was attributed to –C–O– (ether) stretching vibration.31 While peaks at 2910 and 1200 cm−1 are for C–H and C–N stretches respectively. The FTIR spectrum also provided an idea about bio-molecules bearing different functionalities which are present in the underlying system.
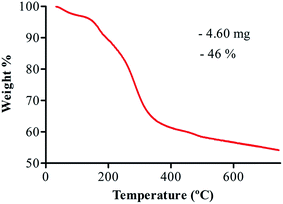
3.4. TGA
The thermal response of AuNPs was investigated with TGA and the results are shown in Fig. 4. The total weight loss observed was about 4.6 mg (46%) for AuNPs. This result indicated that AuNPs prepared from Longan fruit juice were capped with more phytochemicals. Weight loss due to the thermal decomposition of the organic part allowed for calculation of the extract/gold ratio which is in agreement with the effect of the extract/gold ratio on the size of AuNPs (HRTEM).173.5. Anticancer activity of AuNPs
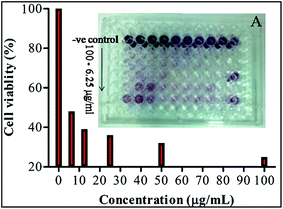
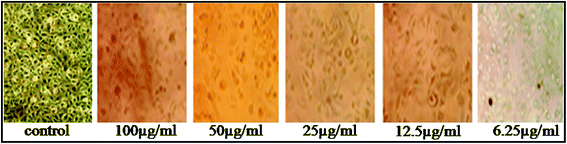
An exhaustive MTT assay on MCF-7 cells was conducted to evaluate anticancer activity of the synthesized AuNPs in different doses. In vitro cytotoxicity of AuNPs against human breast cancer cells MCF-7 showed a significant decrease in cell viability of MCF-7 when the concentration of the bio-synthesized AuNPs was increased from 6.25–100 μg mL−1 (Fig. 5). The presently synthesized AuNPs showed a significant cytotoxic effect against breast cancer cells MCF-7 with a much lower concentration than previously reported biocompatible AuNPs.32,33 Optical microscopy studies of MCF-7 cells showed morphological changes, following the suppression of cell growth and finally cell clumping and death (Fig. 6) due to exposure to biogenic AuNPs. It is clear from Fig. 5 that as the concentration increases the cell viability decreases. At a concentration of 100 μg mL−1, the maximum cytotoxic effect (75%) was observed in MCF-7 cells as evident from Fig. 6 where MCF-7 cells show 25% viability at this concentration. A smaller size (25 nm), spherical morphology, uniform distribution (HRTEM) and the capping phytochemicals may be factors which prove that AuNPs are such an excellent anticancer agent. The recorded cytotoxicity of AuNPs of this study could be due to either increased or decreased expression patterns of both anti-apoptotic Bcl-2 protein and pro-apoptotic Bax protein, as evidenced by an earlier report.34 In fact, AuNPs may induce reactive oxygen species and cause damage to cellular components leading to cell death.35 These results clearly show the significant potential of bio-synthesized AuNPs against cancer cells MCF-7.3.6. Hemolytic activity
The fundamental requirement of a biomaterial is that it must be biocompatible and not generate any toxic effects. Biocompatibility is generally a surface property, and can be determined based on the adverse host response intensity.36 In the present work we have investigated the biocompatibility of AuNPs using a hemolysis assay. The percentage of hemolysis caused by different concentrations of AuNPs on RBCs is shown in (Table 1). It was found that AuNPs have no hemolytic activity against RBCs on different tested concentrations. Gold is nontoxic, inert and stable8 and has a high binding capacity, thereafter AuNPs are considered a potential anti-cancer drug carrier.37| Sample (n = 3) | Hemolytic activity (%) (OD540 nm) |
|---|---|
| a Experiments are in triplicates and the results are presented as mean ± standard deviation. OD540 nm is the optical density at 540 nm. | |
| Control | 1.22 ± 0.18 |
| 1% Triton X-100 | 103 ± 0.0 |
| AuNPs (12.5 μg) | 1.22 ± 0.11 |
| AuNPs (25 μg) | 1.24 ± 0.15 |
| AuNPs (50 μg) | 1.23 ± 0.0 |
| AuNPs (75 μg) | 1.25 ± 0.12 |
| AuNPs (100 μg) | 1.27 ± 0.11 |
| AuNPs (125 μg) | 1.29 ± 0.13 |
3.7. Antioxidant activity of AuNPs
The reducing power of compounds is directly proportional to their antioxidant activity. Antioxidant or antiradical activity is based upon the reduction of 1,1-diphenyl-2-picrylhydrazyl (DPPH). Due to the presence of an odd electron, it gives a strong absorption maximum at 517 nm. As this electron becomes paired off in the presence of a hydrogen donor, i.e. a free radical scavenging antioxidant, the absorption strength at 517 nm is decreased, and the resulting decolorization is stoichiometric with respect to the number of electrons captured.38 The antioxidant activity of AuNPs was assessed using the DPPH free radical scavenging assay with Vit.C as a positive control. DPPH is a stable compound and accepts hydrogen or electrons from AuNPs. The results obtained in the DPPH assay showed effective free radical inhibition by AuNPs (Fig. 7). In this dose dependent assay six different concentrations were used and the activity was found to increase with increasing concentrations of AuNPs. The excellent antioxidant activity may be due to the smaller size, spherical shape, and high and uniform dispersion as clear from HRTEM analysis. The spherical shape and successful dispersion of the AuNPs provide a sufficient surface area and a good number of contact sites. The same results were shown with enhanced DPPH scavenging activity by selenium, platinum, AuNPs39–41 and by torolex and chitosan coated AuNPs.40,42 According to some researchers the antioxidant property has been reported to be related to the development of reducing power. Reductones, which have strong reducing power, are generally believed not only to react directly with peroxides but also to prevent peroxide formation by reacting with certain precursors.43 Antioxidants control the activity of free radicals hence they will support the body’s immune system, allowing the system to significantly deal with viruses and other elements that invade the body.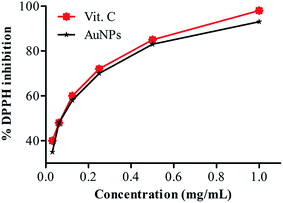 | ||
| Fig. 7 Antioxidant activity of AuNPs showing concomitant increase in % DPPH inhibition with increasing concentration of AuNPs. | ||
3.8. Fluorescence emission
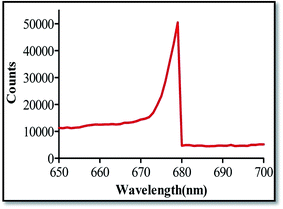
The fluorescence spectrum of biosynthesized AuNPs measured with an excitation wavelength of 555 nm is displayed in Fig. 8. The centre of the photoluminescence (PL) band appears at 679 nm. The incident light at 555 nm led to excitation of the surface plasmon coherent electronic motion as well as the d electrons. The optical properties of gold are due to 5d (valence) and 6sp (conduction) electrons. The outermost d and s electrons of the constituent atoms must be treated together leading to six bands: five of them are fairly flat, lying a few eV below the Fermi level, and are usually denoted as d bands; the sixth one, which is almost free-electron like is known as the conduction band or sp band.44 Single photon luminescence from gold has been described44,45 as a three-step process as follows: (i) excitation of electrons from the occupied d to the sp band which is above the Fermi level to generate electron–hole pairs, (ii) scattering of electrons and holes on a picosecond time scale with partial energy transfer to the phonon lattice and (iii) recombination of an electron from an occupied sp band with the hole resulting in photon emission. The results of this study are of high significance to the field of nanotechnology as AuNPs can be a promising candidate for many biological and biomedical applications.In the bioscience and medical fields, AuNPs are used as immunostaining marker particles for electron microscopy and as chromophores for immunoreactions and nucleic acid hybridization.46,47 The light absorption and emission characteristics of AuNPs are exploited in the detection and treatment of cancer. These properties of biosynthesized AuNPs give them high potential for use in various medical applications; particularly in diagnostics and therapy where they promise increased sensitivity, speed and cost effectiveness.
4. Conclusion
Highly dispersed stable bioactive AuNPs (average size 25 nm) were synthesized using Longan fruit juice as a reducing and stabilizing agent by a bio-green, cost effective and eco-friendly protocol. This single step procedure can be used for large scale production of AuNPs without the use of any external reducing or stabilizing agents as it is environmentally benign and safe for clinical research and could result in economic feasibility. The biomedical applications of the synthesized AuNPs are substantiated by their potent or significant cytotoxicity against human breast cancer cell lines MCF-7. The synthesized AuNPs are found friendly to humans as they show no cytotoxicity against healthy RBCs. AuNPs also possess significant antioxidant activity as they significantly scavenged DPPH free radicals. AuNPs have a high degree of luminescence. This result could be eco-friendly for cancer treatment, cell imaging, biosensors, drug delivery and other medical applications. However, the outcome, transport and accumulation of nanoparticles inside the human body must be thoroughly studied prior to the approval for use as an anticancer drug.Acknowledgements
The authors would like to acknowledge financial support from National Natural Science Foundation of China (no. 21376017 and no. 21176018).References
- T. N. V. K. V. Prasad and E. K. Elumalai, Asian Pac. J. Trop. Biomed., 2011, 1, 439–442 CrossRef CAS PubMed.
- K. N. Thakkar, S. S. Mhatre and R. Y. Parikh, Nanomedicine, 2010, 6, 257–262 CrossRef CAS PubMed.
- Z. Khan, J. I. Hussain and A. A. Hashmi, Colloids Surf., B, 2012, 95, 229–234 CrossRef CAS PubMed.
- K. Vijaymohan, N. S. P. Kamala, N. Udayaprakash and D. Madhankumar, Colloids Surf., B, 2012, 94, 114–117 CrossRef PubMed.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547 CrossRef CAS PubMed.
- M. M. Kemp, A. Kumar, S. Mousa, T. Park, P. Ajayan, N. Kubotera, S. A. Mousa and R. J. Linhardt, Biomacromolecules, 2009, 10, 589 CrossRef CAS PubMed.
- B. Zhang, X. Ye, W. Dai, W. Hou, F. Zuo and Y. Xie, Nanotechnology, 2006, 17, 385 CrossRef CAS.
- W. Cai, T. Gao, H. Hong and J. Sun, Nanotechnol., Sci. Appl., 2008a, 1, 17–32 Search PubMed.
- W. Cai, T. Gao, H. Hong and J. Sun, Cancer Lett., 2008b, 269, 57–66 Search PubMed.
- P. Ghosh, G. Han, M. De, K. C. Kim and M. V. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307–1315 CrossRef CAS PubMed.
- R. E. Thomas and R. A. Papandrea, Med. J. Aust., 1993, 158, 720 CAS.
- J. E. Hutchison, ACS Nano, 2008, 2, 395–402 CrossRef CAS PubMed.
- A. U. Khan, Y. Wei, Z. U. H. Khan, K. Tahir, S. U. Khan, A. Ahmad, F. U. Khan, L. Cheng and Q. Yuan, Int. J. Electrochem. Sci., 2015, 10, 7905–7916 CAS.
- A. Ahmad, F. Syed, A. Shah, Z. Khan, K. Tahir, A. U. Khan and Q. Yuan, RSC Adv., 2015, 5, 73793–73806 RSC.
- K. Tahir, S. Nazir, B. Li, A. U. Khan, Z. U. H. Khan, A. Ahmad and Q. U. Khan, J. Photochem. Photobiol., B, 2015, 153, 261–266 CrossRef CAS PubMed.
- K. Tahir, S. Nazir, B. Li, A. U. Khan, Z. U. H. Khan, P. Y. Gong, S. U. Khan and A. Ahmad, Mater. Lett., 2015, 156, 198–201 CrossRef CAS.
- A. Ahmad, Y. Wei, F. Syed, M. Imran, Z. U. H. Khan, K. Tahir, A. U. Khan, M. Raza, Q. U. Khan and Q. Yuan, RSC Adv., 2015, 5, 99364–99377 RSC.
- K. Tahir, S. Nazir, B. Li, A. U. Khan, Z. U. H. Khan, A. Ahmad and F. U. Khan, Sep. Purif. Technol., 2015, 150, 316–324 CrossRef CAS.
- K. Tahir, B. Li, S. Khan, S. Nazir, Z. U. H. Khan, A. U. Khan and R. U. Islam, J. Alloys Compd., 2015, 651, 322–327 CrossRef CAS.
- A. Suzan, B. A. Mansor, N. Farideh and M. Rosfarizan, Mater. Lett., 2014, 116, 275–277 CrossRef.
- A. K. Mittal, Y. Chisti and U. C. Banerjee, Biotechnol. Adv., 2013, 31, 346–356 CrossRef CAS PubMed.
- D. Mubarak Ali, N. Thajuddin, K. Jeganathan and M. Gunasekaran, Colloids Surf., B, 2011, 85, 360–365 CrossRef CAS PubMed.
- N. Rangkadilok, L. Worasuttayangkurn, R. N. Bennettand and J. Satayavivad, J. Agric. Food Chem., 2005, 53, 1387–1392 CrossRef CAS PubMed.
- W. S. Veda kumari, P. Prabu, S. C. Babu and T. P. Sastry, Biochim. Biophys. Acta, 2013, 1830(8), 4244–4253 CrossRef CAS PubMed.
- C. W. Choi, S. C. Kim, S. S. Hwang, B. K. Choi, H. J. Ahn, M. Y. Lee, S. H. Park and S. K. Kim, Plant Sci., 2002, 163, 1161–1168 CrossRef CAS.
- M. Noginov, G. Zhu, M. Bahoura, J. Adegoke, C. Small, B. Ritzo, V. Drachev and V. Shalaev, Appl. Phys., 2007, 86, 455–460 CrossRef CAS.
- M. Gutierrez and A. Henglein, J. Phys. Chem., 1993, 97, 11368–11370 CrossRef CAS.
- P. Magudapathy, P. Gangopadhyay, B. Panigrahi, K. Nair and S. Dhara, Phys. B, 2001, 299, 142–146 CrossRef CAS.
- S. P. Chandran, M. Chaudhary, R. Pasricha, A. Ahmad and M. Sastry, Biotechnol. Prog., 2006, 22, 577–583 CrossRef CAS PubMed.
- S. S. Shankar, A. Ahmad, R. Pasricha and M. Sastry, J. Mater. Chem., 2003, 13, 1822–1826 RSC.
- P. V. Kamat, Chem. Rev., 1993, 93, 267–300 CrossRef CAS.
- J. B. Punuri, P. Sharma, S. Sibyala, R. Tamuli and U. Bora, Int. Nano Lett., 2012, 2, 18–26 CrossRef.
- P. Joshi, S. Chakraborti, J. E. Ramirez-Vick, Z. A. Ansari, V. Shanker, P. Chakrabarti and S. P. Singh, Colloids Surf., 2012, 95, 195–200 CrossRef CAS PubMed.
- M. E. Selim and A. A. Hendi, Asian Pacific Journal of Cancer Prevention, 2012, 13, 1617–1620 CrossRef PubMed.
- J. P. Jacob, S. S. Finub and A. Narayanan, Colloids Surf., B, 2012, 91, 212–214 CrossRef PubMed.
- J. Upadhyay, A. Kumar, B. Gogoi and A. K. Buragohain, Mater. Sci. Eng., C, 2015, 54, 8–13 CrossRef CAS PubMed.
- G. F. Paciotti, L. Myer, D. Weireich, D. Goia, N. Pavel, R. E. McLaughlin and L. Tamarkin, Drug Delivery, 2004, 11, 3169–3183 CrossRef PubMed.
- R. VennilaRaj, K. Palanisamy, M. Arthanareeswari and S. Bitragunta, Int. J. Chem., 2013, 34(1), 1103–1107 Search PubMed.
- X. Gao, J. Zhang and L. Zhang, Adv. Mater., 2002, 14, 290 CrossRef CAS.
- H. Yin, Free Radicals Biol. Med., 2007, 43, 1229–1230 CrossRef CAS PubMed.
- J. P. Saikia, S. Paul, B. K. Konwar and S. K. Samdarshi, Colloids Surf., B, 2010, 78, 146–148 CrossRef CAS PubMed.
- D. RAuhunandan, M. D. Bedre, S. Basavaraja, B. Sawle, S. Manjunath and A. Venkataraman, Colloids Surf., B, 2010, 79, 235–240 CrossRef PubMed.
- D.-Z. Xia, X.-F. Yu, Z.-Y. Zhu and Z.-D. Zou, Nat. Prod. Res., 2011, 25, 1893–1901 CrossRef CAS PubMed.
- A. Lin, D. H. Son, I. H. Ahn, G. H. Song and W. T. Han, Opt. Express, 2007, 15, 6374–6379 CrossRef CAS PubMed.
- H. Wang, T. B. Huff, D. A. Zweifel, W. He and P. S. Low, et al., Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 15752–15756 CrossRef CAS PubMed.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS PubMed.
- M. Huber, T. F. Wei, U. R. Muller, P. A. Lefebvre and S. S. Marla, et al., Nucleic Acids Res., 2004, 32, 137 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |