DOI:
10.1039/C5RA27085E
(Paper)
RSC Adv., 2016,
6, 39492-39499
CO2 decomposition in a packed DBD plasma reactor: influence of packing materials†
Received
18th December 2015
, Accepted 13th April 2016
First published on 14th April 2016
Abstract
Carbon dioxide (CO2) decomposition has drawn significant interest over the years due to its global warming potential. A packed bed dielectric barrier discharge reactor has been designed and tested for the conversion of CO2 into carbon monoxide (CO). The discharge volume was filled with different packing materials (glass beads, alumina, anatase titania, ceria) so as to understand the influence of dielectric constant, porosity and ultraviolet light. Typical results indicated that the packed bed DBD promotes CO2 conversion into CO and oxygen and CeO2 packing showed the highest conversion (10.6%) at a specific input energy of 4.8 J mL−1. The best performance of CeO2 may be due to oxygen vacant sites, which stabilize the atomic oxygen formed in the reaction and thereby promoting CO2 conversion. During the present study, CO2 decomposition has been achieved at ∼0.139 eV per molecule.
1. Introduction
The contribution of carbon dioxide (CO2) towards the greenhouse effect is well-established and there is an immediate need to address this issue.1 Although various approaches like carbon capture and storage (CCS), carbon capture and utilization (CCU), and catalytic/photocatalytic conversion have been tested, their scope is limited either due to the environmental regulation and/or cost considerations.2,3 When compared with the widely practiced chemical/physical methods, direct decomposition of CO2 into CO has an advantage, in terms of CO formation, which is a feed stock in the chemical industry.2,4 However, the enthalpy change of CO2 decomposition indicates that this process is highly endothermic and demands high operating temperature [eqn (1) and (2)].5| |
 | (1) |
| | |
CO2 → CO + O, ΔH = 5.5 eV per molecular 532 kJ mol−1
| (2) |
Thermal decomposition of CO2 demands temperature excess of 2000 K for the formation of CO and O2.6 Among the alternative processes for CO2 decomposition, non-thermal plasma (NTP) activation has advantages like operation under ambient conditions, presence of energetic electrons capable of initiating the reaction under mild conditions, etc. Various NTP configurations like glow discharge, corona discharge,7–9 microwave discharge,10 radio frequency discharge11 and gliding arc discharge5,12 have been tested for this purpose. Recently, dielectric barrier discharge (DBD) was also tested for CO2 conversion.13–16 DBD plasma generates the high energy electrons (1–10 eV), capable of initiating the chemical reactions while keeping the background under ambient conditions.17–19 In addition to the energetic electrons, DBD also produces UV-Vis light, oxygenated species, etc. Hence, integration of a suitable catalyst/photocatalyst is expected to increase the efficiency of the process. Especially integrating porous solids like alumina improves the interaction of the CO2 with the active species of plasma, and thereby facilitating the vibrational relaxation of the excited CO2 molecules, leading to the dissociation of CO2 molecule under energetically feasible conditions.
In general, the performance of DBD reactor depends on the configuration of the reactor, background gas, flow rate of the gas, catalyst/packing material and the input power.12,20,21 It was reported that among argon, nitrogen and helium, nitrogen is more effective for CO2 decomposition reaction.12 Bogaerts et al., have reported that microwave plasma is energy efficient (23%) than DBD plasma (5%).10 Xin Tu et al., reported that BaTiO3 packing is more effective than glass beads, which was assigned due to the high dielectric constant of BaTiO3, whereas, David Yap et al., reported the best conversion while using the glass beads.6,13 In general, the effect of packing material may either be due to the surface activation (dielectric, porous solids), catalytic action promoted by the discharge (metal oxides)22 and/or by the radiation (TiO2).23–28 However, to understand this, a detailed study is warranted. With this background, the present study is aimed at understanding the influence of packed bed DBD reactor during the CO2 decomposition and to achieve the best CO selectivity. For this purpose, Al2O3 beads were chosen to understand the influence of the surface, whereas TiO2 packing is to understand the influence of the UV-radiation. CeO2 packing is to understand the oxygen storage/release capacity.29,30
2. Experimental set-up
The experimental setup used in the present study has been shown in Fig. 1.
 |
| | Fig. 1 Schematic diagram of Experimental set up. | |
Discharge was generated in a cylindrical DBD reactor, which has 22 and 19 mm outer and inner diameter, respectively. A wire mesh wrapped outside of the quartz tube acts as the outer electrode (9 cm in length), whereas a stainless steel rod of 10 mm diameter serves as the inner electrode. The effective discharge gap is 4.5 mm and discharge volume is ∼73.79 mL. The inner electrode was connected to a high voltage source and the outer electrode was grounded through a capacitor (4 μF) (Fig. 2).
 |
| | Fig. 2 DBD reactor set up. | |
100% CO2 (without dilution) gas was sent through the DBD reactor at a fixed flow rate of 20 mL min−1. The DBD plasma was ignited by varying the AC voltage in the range 14 to 22 kV at a fixed frequency 50 Hz. A high voltage probe (Agilent 34136A HV probe) was used to measure the applied voltage. Charge–voltage (Q and V) signals were measured with a digital oscilloscope (Tektronix TDS2014B) and plotted to get a typical Lissajous figure (Fig. 3), whose area multiplied with frequency gives the power (W), which on dividing with the flow rate gives the energy (J) dissipated in the discharge.
 |
| | Fig. 3 Lissajous figure for DBD reactor as a function of voltage. | |
The CO2 and CO gases were analyzed by using a VARIAN 450 gas chromatography (GC) equipped with a packed column (HAYASEP A, 80/100 mesh, 2 m) and a TCD detector. N2 was used as the carrier gas. The reactor outlet was connected to GC with a long Teflon tube (3 mm diameter) and every 15 minutes an online injection was carried out. Then three injected results were collected corresponding to each voltage and the average area of three gradual injection data has been considered for further calculations. The emission spectrum of the discharge was measured by using an emission spectrometer (Princeton Instrument Action SpectraPro® SP-2300), equipped with three gratings (600 g mm−1 with 500 nm Blaze, 600 g mm−1 with 750 nm Blaze and 1200 g mm−1 with 500 nm Blaze) which uses an optical fiber to collect the spectrum. The emission experiment has been conducted with 600 g mm−1 with 500 nm Blaze. The packed materials used in the present study are: glass beads (3.5–4.5 mm, SD fine-chem limited), TiO2 (3–4 mm, Alfa Aesar), γ-Al2O3 (3–4 mm, Sumitomo Chemical), CeO2 (∼2 mm, Sigma-Aldrich). The surface area of packed materials was estimated by using a NOVA 2200e sorption apparatus. CO2 conversion, CO yield, Specific Input Energy (SIE), energy efficiency and Specific Energy Required per molecule (SER) were calculated by using the following relations:
| |
 | (3) |
| |
 | (4) |
SIE is the energy consumption per mL of the gas flow, which was calculated by using eqn (5).
| |
 | (5) |
| |
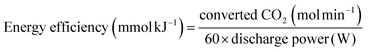 | (6) |
| |
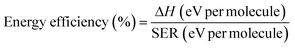 | (7) |
where Δ
H is the enthalpy of reaction
(1) (2.9 eV per molecule for CO formation from CO
2) and SER is the specific energy requirement, which is defined as the total energy consumption per unit of reaction product. SER was calculated by using
eqn (8)| |
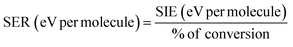 | (8) |
3. Results and discussion
3.1. Effect of packing materials on discharge characteristics
Fig. 3 presents the V–Q Lissajous figure as a function of the voltage, from which the total charge for various packing materials was calculated. As seen in Fig. 3, the area of Lissajous figure and thereby power increases with increasing the voltage. Fig. 4 presents the influence of various packing materials on the discharge characteristics at 22 kV, which indicates that the input power dissipated is different for various packed DBD configurations.
 |
| | Fig. 4 Lissajous figure for different packed material at 22 kV. | |
3.2. Effect of packing material on discharge power
Fig. 5 presents the influence of various packing materials on the power dissipated in the discharge. Among the packing materials studied, TiO2 packing showed the highest power and the discharge power followed the trend: TiO2 > Al2O3 > CeO2 ≈ glass beads > DBD. It is known that materials with high dielectric constant generate more number of microdischarges and create strong electric field at the corners and edges. The dielectric constant for various packed materials follows the order: TiO2 > CeO2 > Al2O3 > glass beads (Table 1). Although CeO2 has higher dielectric constant (25)31,32 than Al2O3, probably due to more uniform shape, the power dissipated is lesser than Al2O3. The power variation data complements the charge variation, as presented in Fig. 6, which also confirms the TiO2-DBD has the highest charge dissipated per half cycle. Fig. 5 and 6 confirm the highest power and charge for TiO2-DBD. Table 2 summarizes the power, specific input energy (SIE) and CO2 conversion for various configurations.
 |
| | Fig. 5 Average power dissipated as a function of the voltage for various packed materials (gas flow rate: 20 mL min−1; without dilution; frequency: 50 Hz). | |
Table 1 The physical properties of packed materials
| Packed material |
Diameter of packed material (mm) |
Surface area (m2 g−1) |
Dielectric constant |
| Glass beads |
3.5–4.5 |
0 |
4.1 |
| TiO2 |
3–4 |
64 |
85 |
| Al2O3 |
3–4 |
133 |
9.1 |
| CeO2 |
2 |
30 |
25 |
 |
| | Fig. 6 Total charge transferred by the micro discharges per half cycle as a function of applied voltage (gas flow rate: 20 mL min−1; without dilution; frequency: 50 Hz). | |
Table 2 CO2 conversion with power and SIE variation for different packed bed DBD
| Voltage (kV) |
Without packing |
Glass beads |
TiO2 |
Al2O3 |
CeO2 |
| Power (W) |
SIE (J mL−1) |
Conv. (%) |
Power (W) |
SIE (J mL−1) |
Conv. (%) |
Power (W) |
SIE (J mL−1) |
Conv. (%) |
Power (W) |
SIE (J mL−1) |
Conv. (%) |
Power (W) |
SIE (J mL−1) |
Conv. (%) |
| 14 |
0.1 |
0.3 |
1.2 |
0.2 |
0.6 |
3.5 |
0.7 |
2.1 |
5.1 |
0.6 |
1.8 |
3.5 |
0.2 |
0.6 |
2.6 |
| 16 |
0.3 |
0.9 |
2.8 |
0.4 |
1.2 |
4 |
1 |
3 |
6.3 |
0.9 |
2.7 |
4.6 |
0.4 |
1.2 |
4.4 |
| 18 |
0.5 |
1.5 |
4.2 |
0.6 |
1.8 |
5.4 |
1.3 |
3.9 |
6.8 |
1.2 |
3.6 |
6.1 |
0.6 |
1.8 |
6.6 |
| 20 |
0.8 |
2.4 |
5.9 |
0.9 |
2.7 |
6.5 |
1.7 |
5.1 |
7.5 |
1.5 |
4.5 |
7.8 |
0.9 |
2.7 |
9.1 |
| 22 |
1.1 |
3.3 |
6.3 |
1.2 |
3.6 |
7.9 |
2.2 |
6.6 |
8.2 |
1.9 |
5.7 |
9.1 |
1.6 |
4.8 |
10.6 |
It is known that dielectric packing improves the electric filed strength, due to which more charge will be transferred to the microdischarges.6 Fig. 6 shows the total charge transferred by the microdischarges per half cycle for various packed bed DBD configurations. For a DBD reactor without any packing, the charge transferred was only between 0.06 μC and 0.51 μC, corresponding to the voltage variation between 14 and 22 kV, respectively. Under the same experimental conditions, 0.41 μC to 0.72 μC was transferred for TiO2 packing. Porous alumina packed DBD showed a slightly lower charge of 0.34 to 0.68 μC, at 14 to 22 kV, respectively, whereas, glass beads and CeO2 packing showed the lowest charge transfer between 0.15 and 0.6 μC. This observation can be explained based on the high dielectric constant (85) of TiO2 beads and alumina (9.1), due to which the strength of microdischarges will be highest for TiO2 than other packing materials.33,34
3.3. Packed bed DBD for carbon dioxide decomposition
Fig. 7 represents the conversion of CO2 as a function of the discharge power for various packed bed configurations. Interestingly, packed bed DBD shows higher conversion than DBD without packing. This may be due to more homogeneous discharge in the presence of packing, surface activation and/or due to improved filed strength. General observation is that the CO2 conversion increases with increasing the input power for all configurations. As shown in Table 2, the discharge power varies in each configuration. With DBD reactor, the maximum discharge power was around 1.1 W at 22 kV, where it showed the conversion of only 6%. Glass beads showed a gradual increase in CO2 conversion with discharge power and the conversion of CO2 reached 8% at 1.2 W. TiO2 packing showed better conversion than DBD and glass beads packing. This may be due to surface activation of the photocatalyst. Since the presence of UV-Vis light is confirmed, it may be assumed that photocatalytic/photoassisted CO2 decomposition may be taking place.35 With TiO2, 8.2% CO2 conversion was observed at ∼2.2 W.
It was reported by Holzer et al., that porous solids like γ-Al2O3 improves the effective residence time of the reactant molecules in the discharge zone and thereby improves the interaction of the reactant molecules with activated species of the discharge.36,37 In order to understand the combined surface area, porosity and dielectric effect, γ-Al2O3 beads (surface area 133 m2 g−1, macroporous and dielectric constant 9.1) were packed. As seen from the Fig. 7, at 1.8 W, 9% conversion was observed. It is worth mentioning that power dissipated was not the same at any voltage (Table 2). For example, at 22 kV, Al2O3 packing shows higher conversion than glass beads. However, at 22 kV, the power dissipated with glass beads packing was only 1.2 W, whereas, it is 1.9 W for Al2O3 packing. The better performance of Al2O3 packed plasma reactor may be due to the high discharge power and enhanced residence time of the gas in the discharge zone due to porous nature of Al2O3. Hence, due to the combined effect of porous nature and dielectric constant, CO2 conversion of ∼9% was observed. This is in agreement with the observations made earlier.9,37
 |
| | Fig. 7 CO2 conversion with respect to discharge power (gas flow rate: 20 mL min−1; without dilution; frequency: 50 Hz). | |
Interesting observation is that CeO2-DBD showed the highest conversion of CO2 at any power. The highest conversion of 10.6% was achieved at 1.6 W (22 kV), whereas, under the same input power conditions, DBD and Al2O3 packed DBD showed only 6.3% and 7.8% conversion, respectively. The best performance of CeO2 packed reactor may be due to its ability to stabilize the nascent oxygen formed during CO2 decomposition. It is worth mentioning that nascent oxygen is a highly reactive species, which can either re-oxidize CO or may recombine to form O2 molecule. It has been reported that CeO2 has oxygen storage and release properties.38 So the nascent oxygen formed due to CO2 decomposition may be stabilized on CeO2 surface and thereby preventing the re-oxidation of CO. It is known that atomic oxygen can promote the decomposition of second CO2 molecule.39 In addition, the carbon was forming due to Boudouard reaction may be oxidized by nascent oxygen. As a result, CO yield also improves with CeO2 packing. As seen from the data presented in Table 2, CO2 conversion followed the trend: DBD < glass beads < TiO2 < Al2O3 < CeO2.
The CO yield with respect to SIE has been shown in Fig. 8. The CO yield also increases with the increasing of SIE. ∼10.5% CO yield achieved with CeO2 packing at 1.6 W, whereas, 6.5% and 5.8% yield was obtained with glass beads and DBD respectively. Al2O3 and TiO2 packing also showed higher CO yield than DBD and glass beads packed DBD. The loosely bound oxygen on CeO2 readily oxidizes the carbon and increases the CO yield.
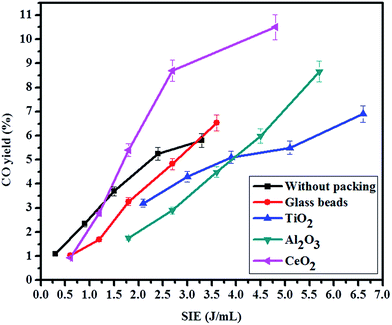 |
| | Fig. 8 Yield of CO with respect to specific input energy (gas flow rate: 20 mL min−1; without dilution; frequency: 50 Hz). | |
3.4. Effect of packed materials on the reaction rate
The CO2 decomposition varies with the packing materials. The decomposition rate constant was calculated by using the following eqn (9).40| | |
ln(Cin/Cout) = (SIE) × K + C
| (9) |
where K is the decomposition rate constant and C is the intercept. Cin and Cout symbolized for CO2 input and CO2 output concentration, respectively.
ln(Cin/Cout) as a function of specific input energy (SIE) for different packing materials have been shown in Fig. 9 and the corresponding decomposition rate constant was 0.020, 0.015, 0.005, 0.016 and 0.018 for CeO2, Al2O3, TiO2, glass beads and DBD, respectively, indicating that CeO2-DBD has the best CO2 decomposition capability.
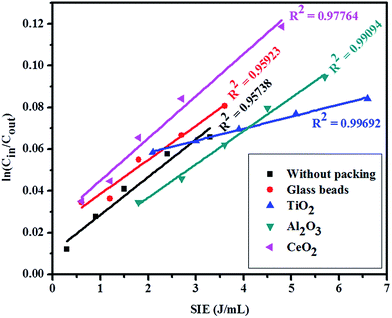 |
| | Fig. 9 Relationship between ln(Cin/Cout) and SIE (linear fits) for different packed materials reactors. | |
3.5. Energy efficiency
Fig. 10 presents the energy efficiency as a function of SIE. It may be concluded that DBD alone is not effective for CO2 decomposition and packed bed DBD improves the energy yield. Energy efficiency decreases on increasing the SIE for all reactors.41 CeO2 packing shows the energy efficiency of 1.91 mmol kJ−1 at SIE 0.6 J mL−1, whereas under the same experimental conditions, TiO2 and Al2O3 shows 1.09 mmol kJ−1 and 0.86 mmol kJ−1, respectively. Interestingly, glass beads packing shows the best energy efficiency (2.58 mmol kJ−1) at the lower SIE (0.6 J mL−1). It is worth mentioning that the observed result with TiO2 packed system shows 30.3% energy efficiency (calculated by eqn. (7)) which is comparable with gliding arc discharge and atmospheric thermal arc reactors which has the energy efficiency of 25% and 30%, respectively.12,39.
 |
| | Fig. 10 Energy efficiency with respect to SIE at different packed materials (gas flow rate: 20 mL min−1; without dilution; frequency: 50 Hz). | |
3.6. Specific energy requirement for decomposition of single CO2 molecule
The specific energy requirement (SER) was calculated by using eqn (8). Fig. 11 shows SER as a function of SIE, which highlights that CeO2 packed DBD reactor demands lowest energy than other configurations. The highest energy demand was 0.187 eV per CO2. CeO2 is also one of the most efficient packing materials, which showed energy requirement of 0.0534 eV per molecule. It signifies that the role of surface is an important factor in CO2 dissociation. CeO2 prevents the recombination of oxygen atoms and/or prevents the CO oxidation to CO2. As a result, CeO2 packed DBD showed the best CO2 conversion. TiO2 and Al2O3 packed reactor demands 0.095 to 0.187 eV per molecule and 0.1194 to 0.1456 eV per molecule, respectively at SIE of 0.488 to 1.534 eV per molecule and 0.418 to 1.325 eV per molecule.
 |
| | Fig. 11 Specific Energy Requirement (SER) with respect to Specific Input Energy (SIE) at different packed materials. | |
3.7. Optical emission spectroscopy
The emission spectrum of the discharge was recorded for various packed DBD reactors to understand the nature of the activate species formed in plasma (Fig. 12). The signature peaks corresponding to excited CO and CO2 were observed. Very intense peak at 335 nm was due to CO2 asymmetric V2–V16 transition indicating that the dissociation may be due to vibrational relaxation.10,23,42
 |
| | Fig. 12 Emission spectra of CO2 plasma. (Applied voltage 18 kV; grating: 600 glue at 500 nm.) | |
Robby Aerts et al. reported that the vibrational excitation is responsible for CO2 splitting in DBD plasma. Peak centered at 297 nm is due to the third positive system of CO, as reported by Martin Kraus et al.23,43 In the present study, CO2+ peak at 290 nm was not detected, probably due to the fast conversion of CO2+ to CO. CH emission peak at 314 nm (C 2∑–X 2Π) may be due to the presence of 0.05% moisture in the CO2 gas. The typical CO2 bands are found at 357 nm and 375 nm, which are assigned due to the transition of V3–V16 and V6–V19 respectively, whereas, the peak found at ∼414 nm may be the transition between V1–V12 and V8–V20 due to CO2+. The peak at 391.2 nm is due to the 1B2–X1∑+ transition of CO2.44 One more peak observed at 435 nm is due to the vibrational transition of CO2. The peaks around 400 and 406 nm are due to the O2 emission.44,45 The peaks observed around 483 nm, 519.8 nm and 559.4 nm are due to CO emission.25
4. Conclusions
DBD plasma assisted conversion of CO2 into CO has been carried out by using a packed DBD plasma reactor. Present study highlights the promising nature of DBD plasma reactor in converting CO2 into CO. The performance of the DBD plasma reactor was found to increase on integrating various packing materials. It has been confirmed that CeO2 packing showed the best performance and appears to be economical. The best CO2 conversion of ∼10.6% has been achieved at 1.6 W, while using CeO2-DBD, which is assigned due to the ability of CeO2 to stabilize the product atomic oxygen and thereby preventing the re-oxidation of CO.
Acknowledgements
The authors gratefully acknowledge to Ministry of New and Renewable Energy, New Delhi, India for supporting to this project (CHY/2014-15/019/MNRE/CHS/0140). Debjyoti thanks UGC India for junior research fellowship.
Notes and references
- IEA, CO2 emissions from fuel combustion Highlights, 2014 Edition Search PubMed
 .
. - R. D. Richardson, E. J. Holland and B. K. Carpenter, A renewable amine for photochemical reduction of CO2, Nat. Chem., 2011, 3, 301–303 CrossRef CAS PubMed
 .
. - F. Studt, I. Sharafutdinov, F. Abild-Pedersen, C. F. Elkjær, J. S. Hummelshøj, S. Dahl, I. Chorkendorff and J. K. Nørskov, Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol, Nat. Chem., 2014, 6, 320–324 CrossRef CAS PubMed
 .
. - C. Nordhei, K. Mathisen, I. Bezverkhyy and D. Nicholson, Decomposition of carbon dioxide over the putative Cubic spinel nanophase cobalt, nickel, and zinc ferrites, J. Phys. Chem. C, 2008, 112, 6531–6537 CAS
 .
. - T. Nunnally, K. Gutsol, A. Rabinovich, A. Fridman, A. Gutsol and A. Kemoun, Dissociation of CO2 in a low current gliding arc plasmatron, J. Phys. D: Appl. Phys., 2011, 44, 274009 CrossRef
 .
. - D. Mei, X. Zhu, Y. L. He, J. D. Yan and X. Tu, Plasma-assisted conversion of CO2 in a dielectric barrier discharge reactor: understanding the effect of packing materials, Plasma Sources Sci. Technol., 2015, 24, 015011 CrossRef CAS
 .
. - G. Horvath, J. D. Skalný and N. J. Mason, FTIR study of decomposition of carbon dioxide in dc corona discharges, J. Phys. D: Appl. Phys., 2008, 41, 225207 CrossRef
 .
. - T. Mikoviny, M. Kocan, S. Matejcik, N. J. Mason and J. D. Skalny, Experimental study of negative corona discharge in pure carbon dioxide and its mixtures with oxygen, J. Phys. D: Appl. Phys., 2004, 37, 64 CrossRef CAS
 .
. - Y. Wen and X. Jiang, Decomposition of CO2 using pulsed corona discharges combined with catalyst, Plasma Chem. Plasma Process., 2001, 21, 665–678 CrossRef CAS
 .
. - T. Kozák and A. Bogaerts, Splitting of CO2 by vibrational excitation in non-equilibrium plasmas: a reaction kinetics model, Plasma Sources Sci. Technol., 2014, 23, 045004 CrossRef
 .
. - Y. F. Wang, C. H. Tsai, M. Shih, L. T. Hsieh and W. C. Chang, Direct Conversion of Methane into Methanol and Formaldehyde in an RF Plasma Environment II: Effects of Experimental Parameters, Aerosol Air Qual. Res., 2005, 5, 211–224 CAS
 .
. - A. Indarto, D. R. Yang, J. W. Choi, H. Lee and H. K. Song, Gliding arc plasma processing of CO2 conversion, J. Hazard. Mater., 2007, 146, 309–315 CrossRef CAS PubMed
 .
. - D. Yap, J. M. Tatibouët and C. Batiot-Dupeyrat, Carbon dioxide dissociation to carbon monoxide by non-thermal plasma, J. CO2 Util., 2015, 12, 54–61 CrossRef CAS
 .
. - Q. Yu, M. Kong, T. Liu, J. Fei and X. Zheng, Characteristics of the decomposition of CO2 in a dielectric packed-bed plasma reactor, Plasma Chem. Plasma Process., 2012, 32, 153–163 CrossRef CAS
 .
. - S. Wang, Y. Zhang, X. Liu and X. Wang, Enhancement of CO2 Conversion Rate and Conversion Efficiency by Homogeneous Discharges, Plasma Chem. Plasma Process., 2012, 32, 979–989 CrossRef CAS
 .
. - M. Babaie, P. Davari, P. Talebizadeh, F. Zare, H. Rahimzadeh, Z. Ristovski and R. Brown, Performance evaluation of non-thermal plasma on particulate matter, ozone and CO2 correlation for diesel exhaust emission reduction, Chem. Eng. J., 2015, 276, 240–248 CrossRef CAS
 .
. - T. Nozaki and K. Okazaki, Non-thermal plasma catalysis of methane: Principles, energy efficiency, and applications, Catal. Today, 2013, 211, 29–38 CrossRef CAS
 .
. - T. Nozaki, A. Ăgıral, S. Yuzawa, J. G. E. H. Gardeniers and K. Okazaki, A single step methane conversion into synthetic fuels using microplasma reactor, Chem. Eng. J., 2011, 166, 288–293 CrossRef CAS
 .
. - H. Taghvaei, A. Jahanmiri, M.
R. Rahimpour, M. M. Shirazi and N. Hooshmand, Hydrogen production through plasma cracking of hydrocarbons: Effect of carrier gas and hydrocarbon type, Chem. Eng. J., 2013, 226, 384–392 CrossRef CAS
 .
. - X. Tu, H. J. Gallon, M. V. Twigg, P. A. Gorry and J. C. Whitehead, Dry reforming of methane over a Ni/Al2O3 catalyst in a coaxial dielectric barrier discharge reactor, J. Phys. D: Appl. Phys., 2011, 44, 274007 CrossRef
 .
. - S. Paulussen, B. Verheyde, X. Tu, C. D. Bie, T. Martens, D. Petrovic, A. Bogaerts and B. Sels, Conversion of carbon dioxide to value-added chemicals in atmospheric pressure dielectric barrier discharges, Plasma Sources Sci. Technol., 2010, 19, 034015 CrossRef
 .
. - T. Nozaki and A. Gutsol, Non-thermal plasma-assisted fuel conversion for green chemistry, J. Phys. D: Appl. Phys., 2011, 44, 270301 CrossRef
 .
. - M. Kraus, W. Egli, K. Haffner, B. Eliasson, U. Kogelschatz and A. Wokaun, Investigation of mechanistic aspects of the catalytic CO2 reforming of methane in a dielectric-barrier discharge using optical emission spectroscopy and kinetic modeling, Phys. Chem. Chem. Phys., 2002, 4, 668–675 RSC
 .
. - S. Mahammadunnisa, E. L. Reddy, D. Ray, C. Subrahmanyam and J. C. Whitehead, CO2 reduction to syngas and carbon nanofibres by plasma-assisted in situ decomposition of water, Int. J. Greenhouse Gas Control, 2013, 16, 361–363 CrossRef CAS
 .
. - S. Kameshima, K. Tamura, Y. Ishibashi and T. Nozaki, Pulsed dry methane reforming in plasma-enhanced catalytic reaction, Catal. Today, 2015, 256, 67–75 CrossRef CAS
 .
. - M. H. Pham, V. Goujard, J. M. Tatibouet and C. Batiot-Dupeyrat, Activation of methane and carbon dioxide in a dielectric-barrier discharge-plasma reactor to produce hydrocarbons—Influence of La2O3/γ-Al2O3 catalyst, Catal. Today, 2011, 171, 67–71 CrossRef CAS
 .
. - X. Tu, H. J. Gallon and J. C. Whitehead, Plasma-assisted reduction of a NiO/Al2O3 catalyst in atmospheric pressure H2/Ar dielectric barrier discharge, Catal. Today, 2013, 211, 120–125 CrossRef CAS
 .
. - S. Mahammadunnisa, P. Manoj Kumar Reddy, B. Ramaraju and C. Subrahmanyam, Catalytic nonthermal plasma reactor for dry reforming of methane, Energy Fuels, 2013, 27, 4441–4447 CrossRef CAS
 .
. - J. Guzman, S. Carrettin and A. Corma, Spectroscopic evidence for the supply of reactive oxygen during CO oxidation catalyzed by gold supported on nanocrystalline CeO2, J. Am. Chem. Soc., 2005, 127, 3286–3287 CrossRef CAS PubMed
 .
. - H. You Kim, H. Mo Lee and G. Henkelman, CO Oxidation Mechanism on CeO2−Supported Au Nanoparticles, J. Am. Chem. Soc., 2012, 134, 1560–1570 CrossRef PubMed
 .
. - J. Lappalainen, D. Kek and H. L. Tuller, High carrier density CeO2 dielectrics—implications for MOS devices, J. Eur. Ceram. Soc., 2004, 24, 1459–1462 CrossRef CAS
 .
. - D. Matsushita, Y. Nishikawa, N. Satou, M. Yoshiki, T. Schimizu, T. Yamaguchi, H. Satake and N. Fukushima, Enhancement of dielectric constant due to expansion of lattice spacing in CeO2 directly grown on Si (111), Jpn. J. Appl. Phys., 2004, 43, 1795 CrossRef CAS
 .
. - AZO materials website; http://www.azom.com/article.aspx?ArticleID=1179.html.
- Accuratus. Aluminium Oxide, Al2O3 website; http://accuratus.com/alumox.html
 .
. - L. G. Devi and G. Krishnamurthy, TiO2-and BaTiO3-assisted photocatalytic degradation of selected chloroorganic compounds in aqueous medium: correlation of reactivity/orientation effects of substituent groups of the pollutant molecule on the degradation rate, J. Phys. Chem. A, 2010, 115, 460–469 CrossRef PubMed
 .
. - E. Kusvuran, A. Samil, O. M. Atanur and O. Erbatur, Photocatalytic degradation kinetics of di-and tri-substituted phenolic compounds in aqueous solution by TiO2/UV, Appl. Catal., B, 2005, 58, 211–216 CrossRef CAS
 .
. - F. Holzer, U. Roland and F. D. Kopinke, Combination of non-thermal
plasma and heterogeneous catalysis for oxidation of volatile organic compounds: Part 1. Accessibility of the intra-particle volume, Appl. Catal., B, 2002, 38, 163–181 CrossRef CAS
 .
. - V. Rico-Pérez and A. Bueno-López, Effect of RhOx/CeO2 calcination on metal-support interaction and catalytic activity for N2O decomposition, Appl. Sci., 2014, 4, 468–481 CrossRef
 .
. - A. Fridman, Plasma chemistry, Cambridge University Press, New York, 2008 Search PubMed
 .
. - W. J. Liang, H. P. Fang, J. Li, F. Zheng, J. X. Li and Y. Q. Jin, Performance of non-thermal DBD plasma reactor during the removal of hydrogen sulfide, J. Electrost., 2011, 69, 206–213 CrossRef CAS
 .
. - R. Snoeckx, Y. X. Zeng, X. Tu and A. Bogaerts, Plasma-based dry reforming: improving the conversion and energy efficiency in a dielectric barrier discharge, RSC Adv., 2015, 5, 29799–29808 RSC
 .
. - X. Tu and J. C. Whitehead, Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Understanding the synergistic effect at low temperature, Appl. Catal., B, 2012, 125, 439–448 CrossRef CAS
 .
. - R. Aerts, T. Martens and A. Bogaerts, Influence of vibrational states on CO2 splitting by dielectric barrier discharges, J. Phys. Chem. C, 2012, 116, 23257–23273 CAS
 .
. - G. Garcia-Cosio, M. Calixto-Rodriguez and H. Martinez, Low-pressure plasma discharge of Ar/N2/CO2 ternary mixture, 29th ICPIG, Cancún, México, July 12–17, 2009, Topic number B6 200954 Search PubMed
 .
. - X. Zhu, X. Gao, C. Zheng, Z. Wang, M. Ni and X. Tu, Plasma-catalytic removal of a low concentration of acetone in humid conditions, RSC Adv., 2014, 4, 37796 RSC
 .
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra27085e |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 



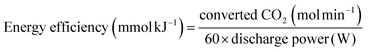
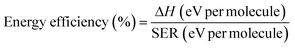
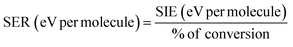







.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.





