Bio-inspired Janus composite nanoscrolls for on-demand tumour targeting†
Abstract
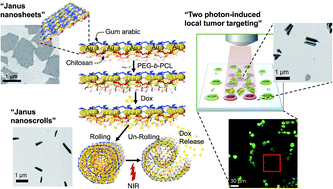
Inspired by the responsive characteristics of natural fibrous counterparts, triple stimuli, pH-, drug-, and near-infrared (NIR) light-responsive Janus composite nanosheets (JCNs) were investigated. The nanosheets consisted of gold islands sequentially hetero-grafted with three different biocompatible polymers (gum arabic, chitosan, and poly(ε-caprolactone)-b-polyethylene glycol (PEG-b-PCL)) that are hierarchically synthesized by a physical deposition–surface functionalization–chemical exfoliation processes. The JCNs are elastic and go through pH-controlled shape recovery activity in a reversible manner. Remarkably, by anchoring the heat-sensitive PEG-b-PCL on the chitosan side of JCNs, the JCNs are securely self-scrolled when doxorubicin molecules are loaded; and unscrolled to release the drug under remotely controlled NIR-irradiation, with negligible premature release, which mimics the structural behavior of a spore launcher in ferns. Moreover, the scrolled JCNs show exceptional photothermal stability under NIR-laser irradiation and the optical hyperthermal effect is capable of inducing death of tumor cells, as well as bright fluorescence and a targeted photothermal anti-tumor effect under two-photon fluorescence imaging. This enables complex nano-therapeutic drug vehicle development in potential cancer theranosis with low toxic side-effects.

 Please wait while we load your content...
Please wait while we load your content...