Solution-processed, inverted organic solar cells with bilayered inorganic/organic electron extraction layers†
Jung Suk Leea,
Myoung Joo Chaa,
Yu Jung Parka,
Jin Young Kim*b,
Jung Hwa Seo*a and
Bright Walker*b
aDepartment of Materials Physics, Dong-A University, Busan 49201, Republic of Korea. E-mail: seojh@dau.ac.kr
bSchool of Energy and Chemical Engineering, Ulsan National Institute of Science and Technology, Ulsan 44610, Republic of Korea. E-mail: jykim@unist.ac.kr; brightium@unist.ac.kr
First published on 7th April 2016
Abstract
In this work, we introduce a solution-processed CdS interlayer for use in inverted bulk heterojunction (BHJ) solar cells, and compare this material to a series of standard organic and inorganic cathode interlayers. Different combinations of solution-processed CdS, ZnO and conjugated polyelectrolyte (CPE) layers were compared as cathode interlayers on indium tin oxide (ITO) substrates to construct inverted solar cells based on poly[[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl][3-fluoro-2-[(2-ethylhexyl)carbonyl]thieno[3,4-b]thiophenediyl]]:[6,6]-phenyl-C71-butyric acid methyl-ester (PTB7:PC71BM) and a poly(3-hexylthiophene):[6,6]-phenyl-C61-butyric acid methyl ester (P3HT:PC61BM) as photoactive layers. Introduction of a CdS interlayer significantly improved the power conversion efficiency (PCE) of inverted PTB7:PC71BM devices from 2.0% to 4.9%, however, this efficiency was still fairly low compared to benchmark ZnO or CPE interlayers due to a low open circuit voltage (VOC). The VOC was greatly improved by introducing a conjugated polyelectrolyte (CPE) layer on top of the CdS layer, yielding outstanding diode characteristics and a PCE of 6.8%. These results indicate that the deep conduction band energy of CdS limits the VOC possible when used as an ETL in polymer fullerene solar cells, however, we have discovered that this issue can be readily overcome via the incorporation of an organic interfacial dipole layer. The best performing interlayer, however, was a single CPE layer alone, which yielded a VOC of 0.727 V, a FF of 63.2%, and a PCE of 7.89%. Using P3HT:PC61BM as an active layer, similar trends were observed. Solar cells without the cathode interlayer yielded a PCE of 0.46% with a poor VOC of 0.197 V and FF of 34.3%. In contrast, the use of inorganic/organic ZnO/CPE interlayers as the cathode interlayer considerably improved the VOC of 0.599 V and FF of 53.3%, resulting in a PCE of 2.99%. Our results indicate that the CdS layer yields excellent diode characteristics, however, performs slightly worse than benchmark ZnO and CPE layers in solar cell devices due to parasitic absorption below 550 nm. These results suggest that layered inorganic/organic interfacial materials are promising candidates as cathode interlayers for high efficiency inverted solar cells through the modification of interfacial contacts.
Introduction
Organic solar cells (OSCs) represent one of several advancing technologies for renewable energy conversion, converting solar energy directly into electricity.1–3 OSCs exhibit desirable mechanical properties including light weight, flexibility, semi-transparency and the potential for large area fabrication process with low cost.4,5 Recently, bulk heterojunction (BHJ) OSCs with an active layer composed of a conjugated polymer as the donor and a fullerene derivative as the acceptor exhibit power conversion efficiencies (PCEs) exceeding 10.8% (ref. 6) for single junction cells, and up to 11.5% (ref. 7) for tandem cells.Conventional BHJ OSC architectures including an active layer sandwiched by a low work function (WF) metal cathode (e.g. Al) and a hole-conducting poly(3,4-ethylene-dioxylenethiophene):poly(styrenesulfonic acid) (PEDOT:PSS) layer on top of an indium tin oxide (ITO) substrate are widely used in the study of OSC devices. However, the conventional structures suffer from poor air stability and device operational lifetime due to the oxidation of the low WF Al, diffusion of oxygen into the active layer through pinholes and grain boundaries in the Al film and the corrosion of ITO by acidic and hygroscopic PEDOT:PSS. Consequently these devices exhibit poor stability and require thorough encapsulation to prevent degradation upon exposure to oxygen and moisture.8–10
Compared with conventional OSCs, inverted device structures using modified ITO as the cathode demonstrate better long-term ambient stability by avoiding acidic PEDOT:PSS and are naturally self-encapsulated because air-stable metals (e.g. Ag and Au) are used as the top electrodes.11,12 The n-type interfacial layer on the ITO substrates is one of the key issues which determine the performance of inverted devices.
Interfacial layers may comprise multi-functional materials that act as selective charge injection layers or modify electrodes, acting to inhibit carrier recombination at the active layer/electrode interface while facilitating the extraction of n-type charge carriers.13–15 Interfacial layers reported so far can be divided into two categories. One is based on organic materials.16 The organic cathode buffer materials were the conjugated polymer (e.g. poly[(9,9-bis(3′-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-2,7-(9,9-dioctylfluore-ne)] (PFN))17 and the conjugated polyelectrolytes (CPEs).10 CPEs, which are conjugated polymers with pendant ionic functionalities (either cationic or anionic), are promising interfacial materials in organic electronic devices. Their solubility in highly polar solvents such as alcohol and water allows simplified device fabrication by avoiding the problem of disturbing neutral organic semiconducting layers that are typically soluble in organic solvents and easily rinse off when multiple layers are deposited. For example, Bazan and coworkers demonstrated conventional solar cell with an electron extracting CPE interlayer which improved the device performance from 5.0 to 6.1%.18,19
The other category of cathode interlayers is based on inorganic materials. In contrast to organic materials, inorganic materials typically exhibit good chemical stability and insolubility in organic solvents, which allows solution processible multi-layer device fabrication.20 Metal oxides such as molybdenum trioxide (MoO3),21 tungsten trioxide (WO3),22 nickel oxide (NiOx),23 and vanadium pentoxide (V2O5)20 with high WF electrodes have been demonstrated as p-type interlayers (promising alternatives to PEDOT:PSS), while cesium carbonate (Cs2CO3),24,25 titanium oxide (TiOx),26 and zinc oxide (ZnO)27 with low WF electrodes have been demonstrated as highly effective n-type interlayers. Among the n-type metal oxides, ZnO is among the most promising candidate as an electron transport layer (ETL), due to its relatively high electron mobility (14 cm2 V−1 s−1),28 environmental stability and high transparency.
In spite of extensive research on interlayer materials, the application of bilayer cathode interfaces, which comprise both organic and inorganic components, is potentially promising but currently lacking.20 Mixed inorganic/organic structures have the advantages of high carrier mobility and stability from inorganic semiconductors together with the advantages of organic materials.29 We recently reported the solution-processible cadmium sulfide (CdS) with a high electron mobility (up to 61 cm2 V−1 s−1);30 given the wide application of n-type CdS layers in inorganic heterojunction solar cells (such as Cu(In,Ga)Se2 and CdTe), CdS constitutes a promising n-type layer for use in inverted OSCs.31,32 CdS is an intrinsically n-type semiconductor with a relatively high carrier concentration, high electron affinity, wide band gap (Eg, 2.4–2.5 eV), relatively high charge carrier mobility and good environmental stability.30,33 All of these properties make CdS attractive as a potential n-type buffer layer of OSCs.
In this work, we have investigated inverted OSC devices using solution-processed inorganic, organic and mixed inorganic/organic interfacial layers and characterized their device performance. Notably, devices with a unique solution-processed CdS film as the cathode interlayer were investigated and compared to benchmark CPE and ZnO ETLs.
Results and discussion
To investigate the influence of various cathode interlayers such as ZnO, CdS and CPE on the device performance, a poly(3-hexylthiophene):[6,6]-phenyl-C61-butyric acid methyl ester (P3HT:PC61BM) and poly[[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl][3-fluoro-2-[(2-ethylh-exyl)carbonyl]thieno[3,4-b]thiophenediyl]]:[6,6]-phenyl-C71-butyric acid methyl-ester (PTB7:PC71BM) were utilized as photoactive layers in our inverted OSCs. The inverted device structure is shown in Fig. 1a, where MoOx/Ag was used as the top anode contact. | ||
| Fig. 1 (a) Diagram illustrating the inverted OSC architecture used: glass/ITO/ETL/active layer/MoOx/Ag and (b) energy level diagram of the component materials used in the devices. | ||
The energy level alignment of each component of the device is shown in Fig. 1b. Since the valence band maximum (VBM) levels of ZnO and CdS are much lower than those of the highest occupied molecular orbitals (HOMOs) of P3HT27 and PTB7,34 these layers serve also as hole-blocking contacts. Similarly, the MoOx layer was used to block electron diffusion to the anode because the conduction band minimum (CBM) of MoOx (2.6 eV) is much higher than the lowest unoccupied molecular orbitals (LUMOs) of the fullerenes.27
The current density–voltage (J–V) characteristics of the inverted PTB7:PC71BM devices with various cathode interfacial layers (CPE, ZnO, CdS, and bilayered ZnO/CPE and CdS/CPE) under illumination of AM 1.5G irradiation with an intensity of 100 mW cm−2 and in the dark are shown in Fig. 2a and b respectively. The photovoltaic parameters are summarized in Table 1. The control device (ITO only) without any interlayer exhibited a poor PCE of 2.03% with a short-circuit current density (JSC) of 15.11 mA cm−2, an open-circuit voltage (VOC) of 0.31 V and a fill factor (FF) of 43.8%. When a CPE was used solely as the interfacial layer, the PCE remarkably increased up to 7.89% with a VOC of 0.73 V, JSC of 17.18 mA cm−2 and FF of 63.2%. Since the VOC of BHJ solar cell is usually determined by the difference in LUMO of acceptor and HOMO of donor, as well as the WF difference between anode and cathode,35,36 the increase in VOC can be attributed to a reduced WF at the cathode after CPE deposition. This reduction in effective WF is mediated by an interfacial dipole effect caused by the CPE layer. Upon depositing a thin CPE film, the ionic functionalities in the CPE orient themselves relative to the film surface in such a way that permanent electronic dipole is formed. This surface dipole reduces the energy which is required to remove an electron from the film surface and thus reduces the effective work function, an effect which has been well documented.10,17
| ETLs | JSC [mA cm−2] | VOC [V] | FF [%] | PCE [%] | RS [Ω cm2] | RSH [MΩ cm2] |
|---|---|---|---|---|---|---|
| ITO | 15.11 | 0.31 | 43.8 | 2.03 | 13.2 | 9.76 × 10−5 |
| CPE | 17.18 | 0.73 | 63.2 | 7.89 | 24.8 | 0.71 |
| ZnO | 15.65 | 0.75 | 66.0 | 7.76 | 4.4 | 4.17 |
| CdS | 14.94 | 0.59 | 55.7 | 4.90 | 51.2 | 4.51 |
| ZnO/CPE | 15.76 | 0.74 | 64.5 | 7.52 | 5.7 | 2.39 |
| CdS/CPE | 15.37 | 0.75 | 59.3 | 6.81 | 15.2 | 44.6 |
The device with the ZnO layer exhibited a PCE of 7.76%, including a VOC of 0.75 V, a JSC of 15.65 mA cm−2 and FF of 66.0%, while the device with CdS layer exhibited a PCE of 4.90% with a VOC of 0.59 V, JSC of 14.94 mA cm−2 and FF of 55.7%. Although the CdS layer has a higher electron mobility than ZnO layer, the results show that it leads to a lower JSC, VOC and FF compared the ZnO or CPE layers. The decrease in VOC is consistent with the deeper conduction band energy of the CdS compared to the ZnO layer. The decrease in VOC is equivalent to a decreased built-in potential across the active layer which results in a leftward shift of the J–V curve, which thus concomitantly acts to decrease the FF. Although the thin (∼30 nm) ZnO and CdS layers have low optical density and high transmittance, they do absorb and reflect a small amount of light compared to the CPE layer. Notably, CdS absorbs a fraction of light below ∼550 nm, thus “eclipsing” some of the incident sunlight and preventing it from reaching the active layer. Thus, the JSC of the CdS and ZnO layers are slightly lower than the ultrathin (<5 nm) CPE layer, which has negligible absorbance. For comparison, devices comprising both inorganic ETLs and CPE layers were investigated. The device with a CPE on the ZnO layer exhibited a PCE of 7.52%, while the use of CPE on the CdS layer resulted in an efficiency of 6.81%, where the JSC and VOC increased to 15.37 mA cm−2 and 0.75 V respectively. Adding the CPE layer to the ZnO ETL had little effect, however, adding the CPE layer to the CdS layer effectively decreased the WF via an interfacial dipole effect and increased the built-in potential, resulting in an optimal VOC. The lower PCE of the CdS/CPE device relative to the ZnO/CPE device is due to a lower FF in this device which in turn is due to a higher series resistance (RS) in the CdS/CPE device.
Fig. 2b shows the J–V characteristics of the device with various cathode interlayers in the dark. The device without any interfacial layer showed the highest leakage current at reverse bias and poor current rectification compared to devices with interfacial layers. The low dark current densities observed from the inverted PTB7:PC71BM devices with interfacial layers at reverse bias indicates that backwards drift/diffusion of charge carriers was suppressed by the interfacial layers. Thus, reduced carrier recombination and improved photocurrent extraction are expected with all of the interfacial layers. More importantly, the low dark current densities at reverse bias and positive cut-in voltages observed from devices with interfacial layers imply that the devices possess larger VOCs than those without interfacial layers. Notably, the cut-in potential at which the dark current rises exponentially is about 0.2 V less for the CdS layer than the other interfacial layers, consistent with the deep conduction band and lower built-in potential and VOC observed in these device.
The enhanced JSC and FF in the devices with interfacial layers imply that RS is smaller than without interfacial layers, while the shunt resistance (RSH) is larger than that without interfacial layers.37 RSH values increased dramatically with all interfacial layers, while RS values varied. The RS of the device with only ZnO was lower than the device with the ZnO/CPE layer (4.4 Ω cm2 and 5.7 Ω cm2 respectively) and the RSH of the device with only ZnO was higher than devices with the ZnO/CPE layer (4.17 MΩ cm2 and 2.39 MΩ cm2 respectively), consistent with the reduced VOC and FF.
In contrast, the RS of the device with only CdS was higher than the device with the CdS/CPE structure (51.2 Ω cm2 and 15.2 Ω cm2 respectively) while the RSH of devices with only CdS was lower than the device with the CdS/CPE structure (4.51 MΩ cm2 and 44.6 MΩ cm2 respectively). This indicates that the addition of a CPE layer to the CdS ETL greatly improves the diode characteristics. It is noteworthy that the device comprising the CdS/CPE layer exhibited the highest RSH by more than an order of magnitude compared to the ZnO, CPE or ZnO/CPE layers, indicating that it might offer the greatest potential as an ETL in photodetector type devices, despite slightly lower performance in photovoltaic devices due to the ultra-low leakage current in these devices.
The external quantum efficiency (EQE) spectra of various devices are shown in Fig. 2c. The EQE spectra for all devices are generally similar, consistent with the identical active layers used in each device, however, the EQE of the device with the CPE ETL only is notably higher than the other curves, consistent with the high JSC observed in this device type relatively high EQE is consistent with the negligible absorption/reflection of this ultra-thin layer and increased transmittance of light to the active layer, as discussed previously. Although the ITO-only device has similar optical properties as the CPE-only device, the reduced quantum efficiency in this condition is consistent with the low built-in potential across the active layer which reduces the efficiency with which charge carriers are extracted. Small differences in the location and intensity of spectral features in the other devices arise from absorption and interference effects due to the optical properties of each ETL.
To confirm the general trends observed in PTB7, based devices, inverted P3HT based solar cells were fabricated using the same series of ETLs. J–V characteristics of the inverted P3HT:PC61BM devices with various cathode interfacial layers under illumination of AM 1.5G irradiation with the intensity of 100 mW cm−2 is shown in Fig. 3. The photovoltaic parameters are summarized in Table 2. The control device (ITO only) without any interfacial layer exhibited a PCE of 0.46% with a JSC of 6.82 mA cm−2, VOC of 0.197 V, and FF of 34.29%. Using the CPE layer alone, the PCE increased significantly to 2.41% with a JSC of 9.73 mA cm−2, VOC of 0.557 V and FF of 44.4%. Also, the device with ZnO layer exhibited a significantly improved PCE of 2.72% with a VOC of 0.585 V, a JSC of 8.97 mA cm−2, and a FF of 51.8%. The devices with CdS layers exhibited a PCE of 1.96% with a VOC of 0.513 V, JSC of 7.88 mA cm−2 and FF of 48.6%. These results show the same trends and substantiate the results observed with PTB7:PC71BM devices, notably the CdS layer yielded a somewhat lower VOC than the other ETLs due to its deep conduction band energy. Devices with the bilayered inorganic/organic interfacial layers exhibited enhanced JSC, VOC and FFs simultaneously, leading to an optimal PCE of 2.99% for devices with the ZnO/CPE bilayer and 2.61% for devices with the CdS/CPE bilayer. The highest PCE was observed using devices with the ZnO/CPE layer among the P3HT:PC61BM devices.
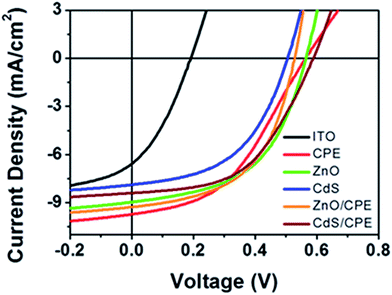 | ||
| Fig. 3 J–V characteristics of inverted P3HT:PC61BM solar cells with various cathode interfacial layers under illumination. | ||
| ETLs | JSC [mA cm−2] | VOC [V] | FF [%] | PCE [%] | |
|---|---|---|---|---|---|
| Average | Best | ||||
| ITO | 6.82 | 0.197 | 34.3 | 0.32 | 0.46 |
| CPE | 9.73 | 0.557 | 44.4 | 2.17 | 2.41 |
| ZnO | 8.97 | 0.585 | 51.8 | 2.46 | 2.72 |
| CdS | 7.88 | 0.513 | 48.6 | 1.67 | 1.96 |
| ZnO/CPE | 9.36 | 0.599 | 53.3 | 2.55 | 2.99 |
| CdS/CPE | 8.40 | 0.594 | 52.4 | 2.08 | 2.61 |
The electronic structures and crystal structures of the interlayers were further investigated by ultraviolet photoelectron spectroscopy (UPS, Fig. 4), X-ray photoelectron spectroscopy (XPS, Fig. S1 ESI†) and X-ray diffraction (XRD, Fig. S2, ESI†). Fig. 4 (left side) shows the secondary electron cut-off of the various interlayers on ITO substrates. The secondary electron cut-off allows accurate determination of the WF of interlayers; by measuring the shift in the edge of these spectra, relative to the substrate (ITO) it is possible to accurately quantify changes in work function and the magnitude of the interfacial dipole effect caused by the interlayer.36
 | ||
| Fig. 4 Secondary electron cut-off and HOMO onset UPS spectra of ITO (black), CPE (red), ZnO (green), CdS (orange), ZnO/CPE (purple) and CdS/CPE (blue) layers spin-cast on the ITO substrates. | ||
According to the UPS measurement, the WF of the UV–ozone treated ITO substrate was 4.8 eV, while the WF of the substrates with interlayers decreased to about from 4.75 eV for the ZnO film or to 4.73 eV for the CdS/CPE film. Due to the dipole moment, the secondary edge is shifted and the WF is decreased, allowing them to function as ETLs. Also, the VBM of the interlayers was determined from the Fermi edge of the UPS spectra, which was located at about 3 eV below the reference Fermi level (EF). Taking into account the Eg obtained from the UV absorption edge, the CBM of the interlayers was found to be about 0.2 eV above EF, indicating the n-type semiconducting property of the spin casted interlayers.
XPS spectra (Fig. S1†) of the CdS films confirmed their compositions, with clear signals arising from photoelectrons ejected from Cd 3d and S 2p orbitals, with binding energies consistent with reference data for CdS. Likewise, ZnO films showed strong photoelectron signals corresponding to Zn 2p and O 1s orbitals, with binding energies consistent with reference values for ZnO. XRD patterns (Fig. S2†) showed broad diffraction peaks with 2θ values and relative intensities which matched greenockite and zincite crystal structures, however, it is clear that the relatively low annealing temperatures and ultra-thin nature of the films led to low crystallinity and weak diffraction patterns.
Atomic force microscopy (AFM) was used to characterize the surface morphology of the interlayers; images sized 2 μm × 2 μm of each cathode interlayer are shown in Fig. 5. Compare to ITO, the CPE film exhibits very small sized grains with a root-mean-square (rms) roughness value of the 1.4 nm as shown in Fig. 5b. The ZnO film displayed in Fig. 5c exhibits a densely packed structure with small grains compared to the CdS film (Fig. 5d). The CdS film shows packed oblong shaped features with relatively large sized grains. The rms roughness values of ZnO and CdS films are 2.2 and 21.0 nm respectively. The images shown in Fig. 5e and f correspond to ZnO and CdS films after depositing CPE interlayers on top, showing that the films become much smoother upon CPE deposition. The rms roughness values of the ZnO and CdS films decreased to 1.6 and 6.7 nm, respectively, upon application of the CPE layer. Such modification of film morphologies can affect the performance of OSCs; particularly, the CdS films are fairly rough for application in OPV devices, and the decreased rms roughness value upon application of the CPE layer corresponds well with the improved device performance. In order to further examine the film structures, their relative surface energies were characterized via water contact angle (θ) (see Fig. S3, ESI†). ITO surfaces exhibited the lowest contact angles (∼37°), while ZnO films were similarly polar (∼39°) and CdS films were less polar (57°). Application of CPE layers increased the contact angle of both ZnO and CdS films to 49 and 69°, respectively, indicating that the CPE interlayers increased the hydrophobicity of the surfaces. This result suggests that the ultra-thin CPE films help to improve the interfacial compatibility between the ionic ZnO and CdS layers and the non-polar active layers which are deposited on top.
 | ||
| Fig. 5 Surface topographic AFM images (size: 2 μm × 2 μm) of (a) ITO, (b) CPE, (c) ZnO, (d) CdS, (e) ZnO/CPE and (f) CdS/CPE films deposited on the ITO substrate. | ||
Conclusions
We have investigated inverted OSC devices using solution-processed inorganic, organic and mixed bilayer inorganic/organic interfacial layers and compared their characteristics. Notably, a new, solution-processed CdS ETL was introduced to explore the applicability of this high-mobility interlayer, which is ubiquitous in inorganic solar cells, in organic devices. All of the cathode interlayers were effectively used with PTB7:PC71BM and benchmark P3HT:PC61BM active layers, demonstrating the broad applicability of the interlayers. The CdS interlayer was observed to provide dramatic improvement relative to devices with no ETL, but yielded somewhat low VOC values compared to ZnO and CPE benchmarks due to a deep conduction band energy. This result is not unexpected, as ZnO and CPE interlayers currently represent some of the highest performing cathode interlayers known in the field. Interestingly, we found that the low VOC observed with the CdS ETL could easily be corrected via the introduction of an interfacial dipole layer and yielded devices with excellent diode characteristics and a PCE of up to 6.8% with a PTB7:PC71BM active layer. The highest PCE of 7.9%, however, was observed with a single, ultra-thin CPE layer which did not introduce any parasitic absorption or reflection losses, as the thicker CdS and ZnO layers did. The CdS/CPE interlayer showed the highest RSH value, indicating strong potential to reduce leakage current in photodetector type devices. Given the success of CdS as an ETL in inorganic solar cells and the scarcity of literature relating to CdS as an ETL in organic electronics, this study reports an important and informative comparison documenting its behaviour in polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fullerene solar cells. These results demonstrate that solution-processed, inverted OSCs can be successfully realized using a variety of organic, inorganic and mixed bilayer cathode interlayers, and that each interlayer has unique properties which may be optimal for specific applications or active materials.
fullerene solar cells. These results demonstrate that solution-processed, inverted OSCs can be successfully realized using a variety of organic, inorganic and mixed bilayer cathode interlayers, and that each interlayer has unique properties which may be optimal for specific applications or active materials.
Experimental
Device fabrication and characterization
Inverted organic solar cell devices were fabricated using the following procedure. First, ITO coated glass substrates were cleaned with detergent, then ultrasonicated in distilled water and isopropyl alcohol, then dried in an oven at 100 °C. ZnO layers were next deposited by diluting a diethylzinc solution (Aldrich, 15 wt% in toluene) with two parts tetrahydrofuran (note: the un-diluted diethyl zinc solution is highly reactive towards air and should be handled inside a glovebox), filtering through a 0.45 μm PTFE syringe filter and spin coating at 3000 rpm for 30 s in air. ZnO layers were then annealed in air on a hot plate at 110 °C for 10 min. The ZnO film thickness was approximately 60 nm. Subsequently, substrates were transferred into a nitrogen filled glove box. CdS films were deposited from solution by spin coating a solution of cadmium t-nonanethiolate (CdTNT, 6 mg mL−1 concentration in chloroform) at 1500 rpm inside a glovebox. These films were dried for 5 min at 150 °C, then annealed at 300 °C for 5 min and gradually cooled down on top of the hotplate. This process was repeated by spin coating another layer of CdTNT, followed by annealing at 150 °C and 300 °C, however, the second annealing step at 300 °C was carried out for 30 min. CPE layers were deposited by spin coating a 0.3% solution of PFN+BIm4− in methanol at 2000 rpm. Active layers were deposited by spin coating a mixed solution of PTB7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1
PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25) in chlorobenzene (concentration of 10 mg mL−1) with 3% DIO additive at 1300 rpm for 60 s on top of the substrate to obtain a BHJ film with thickness of approximately 80 nm. Samples were then brought under vacuum (about 10−7 Torr), and MoOx (≈3.7 nm) and Ag electrodes (100 nm) were deposited on top of the BHJ layer by thermal evaporation. Inverted devices using P3HT:PC61BM in o-dichlorobenzene (o-DCB) were prepared following the same procedure with concentration of 10 mg mL−1 and thickness of approximately 200 nm. Current density–voltage measurements were collected using a Keithley 2635 source measure unit and carried out inside a nitrogen filled glove-box using a high quality optical fiber to guide the light from a xenon arc lamp to the solar cell device. The solar cell devices were illuminated with an intensity of 100 mW cm−2 as calibrated using a standard silicon reference cell. EQE measurements were carried out using a QEX7 system manufactured by PV Measurements, Inc. UPS and XPS experiments were carried out using a Thermo Fischer Scientific ESCALAB 250XI, while XRD patterns were collected using a Bruker AXS D8 Advance diffractometer.
1.25) in chlorobenzene (concentration of 10 mg mL−1) with 3% DIO additive at 1300 rpm for 60 s on top of the substrate to obtain a BHJ film with thickness of approximately 80 nm. Samples were then brought under vacuum (about 10−7 Torr), and MoOx (≈3.7 nm) and Ag electrodes (100 nm) were deposited on top of the BHJ layer by thermal evaporation. Inverted devices using P3HT:PC61BM in o-dichlorobenzene (o-DCB) were prepared following the same procedure with concentration of 10 mg mL−1 and thickness of approximately 200 nm. Current density–voltage measurements were collected using a Keithley 2635 source measure unit and carried out inside a nitrogen filled glove-box using a high quality optical fiber to guide the light from a xenon arc lamp to the solar cell device. The solar cell devices were illuminated with an intensity of 100 mW cm−2 as calibrated using a standard silicon reference cell. EQE measurements were carried out using a QEX7 system manufactured by PV Measurements, Inc. UPS and XPS experiments were carried out using a Thermo Fischer Scientific ESCALAB 250XI, while XRD patterns were collected using a Bruker AXS D8 Advance diffractometer.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF-2013R1A1A2011591 and 2014R1A1A1037729). This research was supported by the MOTIE (Ministry of Trade, Industry & Energy (#10051954)) and KDRC (Korea Display Research Corporation) support program for the development of future devices technology for display industry.Notes and references
- S. Loser, B. Valle, K. A. Luck, C. K. Song, G. Ogien, M. C. Hersam, K. D. Singer and T. J. Marks, Adv. Energy Mater., 2014, 4, 1301938–1301945 Search PubMed.
- J. W. Jung, F. Liu, T. P. Russell and W. H. Jo, Adv. Mater., 2015, 27, 7462–7468 CrossRef CAS PubMed.
- X. Ouyang, R. Peng, L. Ai, X. Zhang and Z. Ge, Nat. Photonics, 2015, 9, 520–525 CrossRef CAS.
- L. Lu and L. Yu, Adv. Mater., 2014, 26, 4413–4430 CrossRef CAS PubMed.
- K. Zhang, Z. Hu, R. Xu, X.-F. Jiang, H.-L. Yip, F. Huang and Y. Cao, Adv. Mater., 2015, 27, 3607–3613 CrossRef CAS PubMed.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 5293 CrossRef CAS PubMed.
- C.-C. Chen, W.-H. Chang, K. Yoshimura, K. Ohya, J. You, J. Gao, Z. Hong and Y. Yang, Adv. Mater., 2014, 26, 5670–5677 CrossRef CAS PubMed.
- A. K. K. Kyaw, D. H. Wang, V. Gupta, J. Zhang, S. Chand, G. C. Bazan and A. J. Heeger, Adv. Mater., 2013, 25, 2397–2402 CrossRef CAS PubMed.
- Z. Lin, J. Chang, J. Zhang, C. Jiang, J. Wu and C. Zhu, J. Mater. Chem. A, 2014, 2, 7788–7794 CAS.
- H. Choi, J. S. Park, E. Jeong, G.-H. Kim, B. R. Lee, S. O. Kim, M. H. Song, H. Y. Woo and J. Y. Kim, Adv. Mater., 2011, 23, 2759–2763 CrossRef CAS PubMed.
- S. Das, J. K. Keum, J. F. Browning, G. Gu, B. Yang, O. Dyck, C. Do, W. Chen, J. Chen, I. N. Ivanov, K. Hong, A. J. Rondinone, P. C. Joshi, D. B. Geohegan, G. Duscher and K. Xiao, Nanoscale, 2015, 7, 15576–15583 RSC.
- X. Li, F. Xie, S. Zhang, J. Hou and W. Ch Choy, Light: Sci. Appl., 2015, 4, e273 CrossRef CAS.
- H.-C. Chen, S.-W. Lin, J.-M. Jiang, Y.-W. Su and K.-H. Wei, ACS Appl. Mater. Interfaces, 2015, 7, 6273–6281 CAS.
- H.-L. Yipa and A. K.-Y. Jen, Energy Environ. Sci., 2012, 5, 5994–6011 Search PubMed.
- T. Kirchartz, T. Agostinelli, M. C. Quiles, W. Gong and J. Nelson, J. Phys. Chem. Lett., 2012, 3, 3470–3475 CrossRef CAS PubMed.
- Z. B. Henson, Y. Zhang, T.-Q. Nguyen, J. H. Seo and G. C. Bazan, J. Am. Chem. Soc., 2013, 135, 4163–4166 CrossRef CAS PubMed.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591–595 Search PubMed.
- J. H. Seo and T.-Q. Nguyen, J. Am. Chem. Soc., 2008, 130, 10042–10043 CrossRef CAS PubMed.
- J. H. Seo, A. Gutacker, Y. Sun, H. Wu, F. Huang, Y. Cao, U. Scherf, A. J. Heeger and G. C. Bazan, J. Am. Chem. Soc., 2011, 133, 8416–8419 CrossRef CAS PubMed.
- Y. Wu, W. Zhang, X. Li, C. Min, T. Jiu, Y. Zhu, N. Dai and J. Fang, ACS Appl. Mater. Interfaces, 2013, 5, 10428–10432 CAS.
- W. Qiu, R. Muller, E. Voroshazi, B. Conings, R. Carleer, H.-G. Boyen, M. Turbiez, L. Froyen, P. Heremans and A. Hadipour, ACS Appl. Mater. Interfaces, 2015, 7, 3581–3589 CAS.
- K. Wang, Y. Shi, Q. Dong, Y. Li, S. Wang, X. Yu, M. Wu and T. Ma, J. Phys. Chem. Lett., 2015, 6, 755–759 CrossRef CAS PubMed.
- W. Zhang, Z. Tan, D. Qian, Q. Xu, L. Li, S. Li, F. Wang, H. Zheng and Y. Li, J. Appl. Polym. Sci., 2013, 128, 684–690 CrossRef CAS.
- Z.-S. Ma, Q.-K. Wang, C. Li, Y.-Q. Li, D.-D. Zhang, W. Liu, P. Wang and J.-X. Tang, Opt. Commun., 2015, 356, 541–545 CrossRef CAS.
- H. P. Kim, A. R. Bin, M. Yusoff, H. J. Lee, S. J. Lee, H. M. Kim, G. J. Seo, J. H. Youn and J. Jang, Nanoscale Res. Lett., 2014, 9, 323 CrossRef PubMed.
- T. Kuwabara, K. Yano, T. Yamaguchi, T. Taima, K. Takahashi, D. Son and K. Marumoto, J. Phys. Chem. C, 2015, 119, 5274–5280 CAS.
- Y. Sun, J. H. Seo, C. J. Takacs, J. Seifter and A. J. Heeger, Adv. Mater., 2011, 23, 1679–1683 CrossRef CAS PubMed.
- H. Bong, W. H. Lee, D. Y. Lee, B. J. Kim, J. H. Cho and K. Cho, Appl. Phys. Lett., 2010, 96, 192115 CrossRef.
- L. Hu, F. Wu, C. Li, A. Hu, X. Hu, Y. Zhang, L. Chen and Y. Chen, Macromolecules, 2015, 48, 5578–5586 CrossRef CAS.
- B. Walker, G.-H. Kim, J. Heo, G. J. Chae, J. Park, J. H. Seo and J. Y. Kim, RSC Adv., 2014, 4, 3153–3157 RSC.
- C.-H. Hsieh, Y.-J. Cheng, P.-J. Li, C.-H. Chen, M. Dubosc, R.-M. Liang and C.-S. Hsu, J. Am. Chem. Soc., 2010, 132, 4887–4893 CrossRef CAS PubMed.
- S. M. Lee, D. H. Yeon, B. C. Mohanty and Y. S. Cho, ACS Appl. Mater. Interfaces, 2015, 7, 4573–4578 CAS.
- J.-J. Zhu, Z.-Q. Xu, G.-Q. Fan, S.-T. Lee, Y.-Q. Li and J.-X. Tang, Org. Electron., 2011, 12, 2151–2158 CrossRef CAS.
- H. Choi, H.-B. Kim, S.-J. Ko, J. Y. Kim and A. J. Heeger, Adv. Mater., 2015, 27, 892–896 CrossRef CAS PubMed.
- D. Baran, S. E. Ela, A. Kratzer, T. Ameri, C. J. Brabec and A. Hirsch, RSC Adv., 2015, 5, 64724–64730 RSC.
- C. J. Brabec, A. Cravino, D. Meissner, N. S. Sariciftci, T. Fromherz, M. T. Rispens, L. Sanchez and J. C. Hummelen, Adv. Funct. Mater., 2011, 11, 374–380 CrossRef.
- T. Yang, M. Wang, C. Duan, X. Hu, L. Huang, J. Peng, F. Huang and X. Gong, Energy Environ. Sci., 2012, 5, 8208–8214 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Water contact angle. See DOI: 10.1039/c5ra27077d |
| This journal is © The Royal Society of Chemistry 2016 |

