Photoswitchable aggregation-induced emission polymer containing dithienylethene and tetraphenylethene moieties†
Abstract
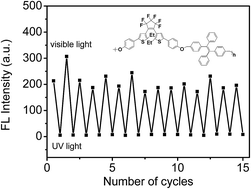
A photochromic aggregation-induced emission (AIE) active polymer containing dithienylethene (DTE) and tetraphenylethene (TPE) moieties was synthesized through a nucleophilic substitution reaction between 1,2-bis[5′-(4′′-hydroxyphenyl)-2′-ethylthien-3′-yl]perfluorocyclopentene and 1,2-bis[4-(bromomethyl)phenyl]-1,2-diphenylethene. The prepared polymer is soluble in various organic solvents and can be easily processed into thin film. The DTE chromophores in the polymer can undergo reversible photoisomerization between their open and closed forms upon irradiation with UV and visible light. Thus, the fluorescence of the prepared AIE polymer can be photoswitched.

 Please wait while we load your content...
Please wait while we load your content...