Surfactant-assisted synthesis of nanoporous nickel sulfide flakes and their hybridization with reduced graphene oxides for supercapacitor applications†
Abstract
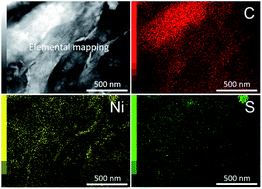
We report a simple soft-templating strategy for the synthesis of nanoporous crystalline nickel sulfide with two-dimensional (2-D) morphology. The nickel sulfide phases and morphologies are varied by changing the hydrothermal temperatures applied. Furthermore, the nanoporous nickel sulfide (PNS) flakes can be hybridized with reduced graphene oxide (rGO) sheets. As compared to bare PNS flakes, the PNS/rGO composite, containing 40% rGO, exhibits a superior electrochemical performance in terms of specific capacitance and cyclic stability. The specific capacitance of this composite is evaluated by a three-electrode system, and it shows the highest specific capacitance of 1312 F g−1 at a scan rate of 5 mV s−1. In addition, this composite is also assembled to form an asymmetric supercapacitor with zeolitic imidazolate framework (ZIF-8)-derived carbon as a negative electrode, which gives a highest specific capacitance of 47.85 F g−1 at 2 A g−1, a high energy density of 17.01 W h kg−1, and a high power density of 10 kW kg−1.

 Please wait while we load your content...
Please wait while we load your content...