DOI:
10.1039/C5RA26932F
(Paper)
RSC Adv., 2016,
6, 40567-40576
Twist fibrous structure of CS–SnO2–PANI ternary hybrid composite for electrochemical capacitance performance
Received
16th December 2015
, Accepted 31st March 2016
First published on 4th April 2016
Abstract
In this study, a twisted fibrous CS–SnO2–PANI ternary hybrid composite structure was synthesized via a two step method; the CS–SnO2 hybrid composite was prepared by a simple chemical precipitation method and the resulting CS–SnO2 suspension was coated with PANI by in situ chemical oxidative polymerization of aniline monomer in acidic medium using ammonium persulphate as the oxidant. The resulting respective materials of chitosan, SnO2 and PANI were characterized by using Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy, X-ray diffraction analysis (XRD) and high resolution scanning electron microscopy (HR-SEM) with energy dispersive X-ray analysis (EDAX), field emission gun-transmission electron microscope (FEG-TEM), thermo-gravimetric (TG) analysis and Brunauer–Emmett–Teller (BET) analysis. The electrochemical capacitors performances were characterized by cyclic voltammetry (CV) and impedance spectroscopy. The electrochemical results demonstrated that the CS–SnO2–PANI ternary hybrid composite achieved 179.20 F g−1 capacitance values in 1 M H2SO4 solution at a 10 mV s−1 potential sweep rate with a maximum of energy density of 8.96 (W h kg−1) and power density of 32.25 (kW kg−1). A synergistic effect among the three components of the chitosan based ternary hybrid composite can be a promising modified electrode material for capacitor applications.
1. Introduction
Electrochemical capacitors have received great attention due to their bridge between conventional capacitors with short charge–discharge times and batteries with long discharge times. There are two promising candidates as electrode materials for supercapacitors: the high surface area carbon materials that exhibit interfacial double layer capacitance with electric charge storage and pseudocapacitors. Conducting polymers and metal oxides are two types of spacers and pseudocapacitive materials used as supercapacitors, but they are limited due to their high electrical resistance and poor cycle stability for supercapacitors.1–5
Among the several conducting polymers, polyaniline (PANI), polypyrrole (PPy) and polythiophene (PTh) have been used for detecting toxic gases.6 PANI has attracted considerable attention because of the easy preparation, low operating temperature, lower cost than other conducting polymers, favourable redox properties, high environmental stability and good controllable electrical and optical properties.7 The potential applications of PANI include organic light weight batteries,8 microelectronic devices, sensors, anticorrosive coatings9 and excellent pseudocapacitors.10
Chitosan is an abundant natural biopolymer with superior characteristics such as biocompatibility, high mechanical strength, low cost, chemically inertness, biodegradability and excellent film forming ability. Over recent years, hybrid composite materials based on chitosan have been developed with metal oxides, conducting polymers and metal nanoparticle due to the simultaneous excellent properties of individual components and outstanding synergistic effect.11 Tin oxide (SnO2), one of the most promising materials, is an n-type semiconductor with a wide band gap (Eg = 3.6 eV) and high capacitance, low cost and with a wide range of applications such as energy storage, gas-sensing materials and antireflecting coatings in solar cells.12,13
In recent years, many researchers were interested in the synthesis of different composites using chitosan, metal oxides and polyaniline based materials, such as chitosan–ZnO,14 chitosan–TiO2,15 chitosan–copper oxide,16 chitosan–SnO2,17 chitosan–CdS,18 chitosan–polyaniline,19 polyaniline/black TiO2,20 polyaniline-coated SiO2,21 PANI–ZnO,22 PANI–SnO2,23 and ternary hybrid composites of chitosan/ZnO–SnO2,24 chitosan–ZnO/PANI,25 Cu–chitosan/alumina nano composite,26 PANI/ZnO–SnO2 composite,27 sulfated β-cyclodextrin/PVP/MnCO3 composite,28 LiCo4 doped chitosan/starch blend electrolyte,29 chitosan based gel electrolyte,30 graphene–SnO2/PEDOT31 and carbon–PANI32 were reported. However, to the best of our knowledge, there is no report about the synthesis of chitosan based SnO2 and PANI ternary hybrid composites.
In the present study, a CS–SnO2–PANI ternary hybrid composite was successfully synthesized via in situ polymerization of aniline, HCl and APS in the presence of CS–SnO2 hybrid composite prepared by a simple chemical precipitation method. The ternary hybrid composites were characterized for functional groups by FTIR and Raman analysis. The crystallite size was calculated by XRD analysis. Surface morphological structures were evaluated by HR-SEM with EDAX and FEG-TEM analysis. Thermal properties were studied by TG analysis and the surface area was determined by BET analysis. Electrochemical properties were analyzed using cyclic voltammetry and electrochemical impedance spectroscopy.
2. Experimental section
2.1. Materials
Chitosan (average molecular weight of 180 kDa and 90% deacetylation) was purchased from M/s South India Sea foods, Kochi, Kerala, India. Aniline monomer was distilled to remove impurities and was stored below 0 °C. Merck Chemical Ltd., Mumbai, India supplied ammonium peroxodisulfate (APS), tin(IV) chloride (98%) was purchased LOBA Chemie (P) Ltd., and acetic acid (99.7%), sodium hydroxide (98%) and hydrochloric acid (95%) were purchased from Fischer Chemical Ltd., Chennai; all were of an analytical reagent grade and used without any further purification. Millipore water was used for all experiments.
2.2. Preparation of materials
Synthesis of chitosan–polyaniline (CS–PANI) composite. CS–PANI composite was prepared using a chemical oxidation and precipitation method that was previously reported with some modifications.25 In this method, CS colloidal solution was first prepared using 0.25 g of CS in 25 mL acetic acid (5%) was well mixed with an ultrasonic cleaner bath (MAXSELL ISO 9001:2008) for 30 minutes. In second step, to prepare CS–PANI composite: 0.9 mL of aniline was dissolved in 25 mL of 1.0 M HCl and the reaction vessel was kept in an ice bath (0–5 °C) for 30 minutes with magnetic stirring and the colloidal CS solution was added to the abovementioned solution and stirring was continued for 1 h followed by quickly adding a 0.25 M APS solution that was dissolved in 25 mL of 1.0 M HCl into the abovementioned solution. During the addition, the solution slowly turned to an emerald green colour. The color was confirmation of aniline monomer polymerization. The reaction vessel was continuously stirred for 4 h and maintained below 5 °C to ascertain the reaction completion. It was then subjected to neutralization with the addition of 50 mL of freshly prepared NaOH (1 M) solution. Then, the precipitate was allowed to settle for 24 h at room temperature and the supernatant solution was discarded. The residue was washed several times with Millipore water, filtered with a suction pump (Tarsons Rockwac Oil-free vacuum pump, model-300), and dried in an oven at 80 °C for 12 h, and designated as CS–PANI.
Synthesis of chitosan–tin oxide–polyaniline (CS–SnO2–PANI) ternary hybrid composite. First a CS–SnO2 suspension was made from 0.25 g of CS in 25 mL acetic acid (5%) being added drop wise with an ultrasonic cleaner bath for 30 minutes and then addition of 25 mL of tin chloride (0.25 M) solution with continuous stirring 2 h at 100 °C. Finally, 25 mL of freshly prepared; NaOH (1 M) solution was added in dropwise until the solution became a pale yellow viscous solution that turned to a white precipitate. The precipitate was continuously stirrer for 2 h using magnetic stirring, then was allowed to settle for 24 h at room temperature and supernatant solution was discarded. The residue was washed several times with Millipore water and the suspension was collected in a beaker labeled CS–SnO2. Subsequently, 0.9 mL of aniline was dissolved in 25 mL of 1.0 M HCl and the reaction vessel was kept in an ice bath (0–5 °C) for 30 minutes with magnetic stirring and the CS–SnO2 suspension was added with continuous stirring for 1 h, followed by quickly adding 0.25 M APS dissolved in 25 mL of 1.0 M HCl that was quickly added into the abovementioned solution. During the addition, the solution slowly turned to an emerald green colour. The color change was confirmation of the aniline monomer polymerizing. The reaction vessel was continuously stirred for 4 h and maintained below 5 °C to ascertain the reaction completion. Settling was allowed for 24 h at room temperature and the supernatant solution was discarded. The residue was washed several times with Millipore water and filtered using a suction pump and dried in an oven at 80 °C for 12 h, and was designated as CS–SnO2–PANI. In a similar manner, CS–SnO2 composite was prepared using same procedure without the polymerization step. PANI was prepared by chemical polymerization of aniline with APS and HCl was used as the initiator and doping agent, respectively, for comparison purposes (Scheme 1).
 |
| | Scheme 1 Schematic of the CS–SnO2–PANI ternary hybrid composite preparation process. | |
2.3. Materials characterization
The functional groups of chitosan, tin-oxide and polyaniline hybrid composites were analyzed using a Fourier transform infrared spectrophotometer (FT/IR-4600 type A, Detector-TGS using KBr pellets). Raman spectroscopy was obtained in the range from 100 to 5000 cm−1. X-ray diffraction (XRD) patterns were studied using an X-ray diffractometer (model XPERT-PRO) (Rigaku diffractor with Cu Kα radiation), (k = 1.5406 Å) operating at 40 kV and 30 mA. High resolution scanning electron microscopy (HR-SEM) was performed with a FEI quanta FEG 250 (instrument) with EDAX and a field emission gun-transmission electron microscope 300 kV (FEG-TEM 300 kV) to study the surface morphology and hybrid composites elemental composition. The specific surface area was determined from a nitrogen adsorption/desorption isotherm using a Gemini model 2380. Thermal stability and degradation behavior of the hybrid composites were analyzed with a thermogravimetric-differential thermal analyzer (model: STA 409 PC/PG, NETZSCH). Approximately, 10 mg of sample was placed in an alumina crucible and the test was performed in the presence of a N2 atmosphere from 35 to 800 °C with a 10 °C min−1 heating rate. The electrical properties were studied using a CH instrument model CHI1102A, in which platinum and saturated calomel electrodes (SCE) was used as the counter and reference electrodes, respectively. The working electrode (GC diameter 3 mm) was prepared from slurry containing 90 wt% of active material, 5 wt% of acetylene block as the conducting material, 5 wt% of poly vinylidene fluoride (PVdF) binder and NMP as the solvent. The slurry was coated onto a glassy carbon electrode and dried.
3. Result and discussions
3.1. FT-IR spectroscopy
The functional groups interaction between chitosan, SnO2 and PANI surface was studied using Fourier transform infrared spectroscopy. The FTIR spectra of PANI, CS–SnO2, CS–PANI and CS–SnO2–PANI hybrid composites in KBr pellet are shown in Fig. 1a. The characteristic PANI bands of PANI at 1569 and 1483 cm−1 is identified as the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching modes of the quinoid and benzenoid rings. The peaks at 1299, 1118 and 804 cm−1 are attributed to the stretching vibrations of benzenoid rings (C–N, C
C stretching modes of the quinoid and benzenoid rings. The peaks at 1299, 1118 and 804 cm−1 are attributed to the stretching vibrations of benzenoid rings (C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–C, respectively).33 The CS–SnO2 composite had obtained two strong peaks at 658 and 567 cm−1 that are ascribed to the Sn–O–Sn vibration in the SnO2 particles. The main characteristic chitosan functional group was observed at 1633 cm−1 due to the bending vibration of –NH2 group in the glucopyranose ring of chitosan.34 The location of the CS–PANI main characteristic peaks are in good agreement with the literature.17,19 This broadening of the absorbed bands between 2918 and 3428 cm−1 was due to the H-bonding interaction between chitosan and PANI. The characteristic bands at 1565 and 1485 cm−1 are the C
N and C–C, respectively).33 The CS–SnO2 composite had obtained two strong peaks at 658 and 567 cm−1 that are ascribed to the Sn–O–Sn vibration in the SnO2 particles. The main characteristic chitosan functional group was observed at 1633 cm−1 due to the bending vibration of –NH2 group in the glucopyranose ring of chitosan.34 The location of the CS–PANI main characteristic peaks are in good agreement with the literature.17,19 This broadening of the absorbed bands between 2918 and 3428 cm−1 was due to the H-bonding interaction between chitosan and PANI. The characteristic bands at 1565 and 1485 cm−1 are the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the quinoid and benzenoid rings, respectively. The band at 1301 cm−1 is the aromatic C–N stretching vibration, 1112 cm−1 is assigned to the N
C stretching vibration of the quinoid and benzenoid rings, respectively. The band at 1301 cm−1 is the aromatic C–N stretching vibration, 1112 cm−1 is assigned to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration and 810 cm−1 is the aromatic C–H out of plane bending vibration.27 The CS–SnO2–PANI hybrid composites show that all characteristic bands of CS–PANI appeared between 400 and 1600 cm−1 region and these were all found in the hybrid composites, although the relative intensity of some bands had shifted into higher wavenumber compared to CS–PANI due to the presence of SnO2. This shift may be ascribed to the formation of hydrogen bonding between the SnCl4 and N–H group of CS–PANI on the surface of the Sn(OH)4 particles.25 It is interesting to note that adding SnCl4 reduced the intensity of the quinine and benzene bands to be much weaker than that in CS–PANI and a new strong absorption peak at 501 cm−1 was attributed to the Sn–O–Sn bond vibrations of the Sn–O–Sn bonds in SnO2. This indicates that amine and imine (N–H and N
N vibration and 810 cm−1 is the aromatic C–H out of plane bending vibration.27 The CS–SnO2–PANI hybrid composites show that all characteristic bands of CS–PANI appeared between 400 and 1600 cm−1 region and these were all found in the hybrid composites, although the relative intensity of some bands had shifted into higher wavenumber compared to CS–PANI due to the presence of SnO2. This shift may be ascribed to the formation of hydrogen bonding between the SnCl4 and N–H group of CS–PANI on the surface of the Sn(OH)4 particles.25 It is interesting to note that adding SnCl4 reduced the intensity of the quinine and benzene bands to be much weaker than that in CS–PANI and a new strong absorption peak at 501 cm−1 was attributed to the Sn–O–Sn bond vibrations of the Sn–O–Sn bonds in SnO2. This indicates that amine and imine (N–H and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) group) nitrogen atoms bonded with Sn4+ via either protonation or coordination complexation. Thus, results indicate the interfacial interaction between CS–PANI and the inorganic SnO2 particle counterpart of the SnO2 particles.
group) nitrogen atoms bonded with Sn4+ via either protonation or coordination complexation. Thus, results indicate the interfacial interaction between CS–PANI and the inorganic SnO2 particle counterpart of the SnO2 particles.
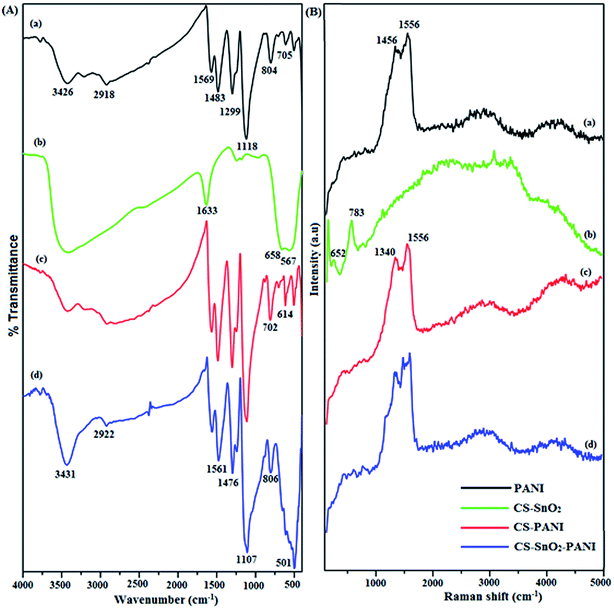 |
| | Fig. 1 (A) FTIR and (B) RAMAN spectra of (a) PANI, (b) CS–SnO2, (c) CS–PANI and (d) CS–SnO2–PANI ternary hybrid composites. | |
3.2. Raman spectroscopy
Raman spectra of PANI, CS–SnO2, CS–PANI and CS–SnO2–PANI hybrid composites are shown in Fig. 1b. The oxidation state of PANI, which possessed the characteristic band at 1456 cm−1 due to the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) stretching vibrations of the benzenoid rings and at 1556 cm−1 are attributed to C
C) stretching vibrations of the benzenoid rings and at 1556 cm−1 are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations of the quinonoid units.35 The CS–SnO2 had a band around 576 cm−1 that is ascribed to Sn–O stretching mode and the peak centered at 771 cm−1 can be assigned to the SnO2 mode, which are related to the expansion and contraction vibrational modes of the Sn–O bonds in rutile SnO2.36 The CS–PANI spectrum shows representative peaks at 1340 and 1556 cm−1, corresponding to the C
N stretching vibrations of the quinonoid units.35 The CS–SnO2 had a band around 576 cm−1 that is ascribed to Sn–O stretching mode and the peak centered at 771 cm−1 can be assigned to the SnO2 mode, which are related to the expansion and contraction vibrational modes of the Sn–O bonds in rutile SnO2.36 The CS–PANI spectrum shows representative peaks at 1340 and 1556 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations of the benzenoid and quinonoid units in the oxidized polyaniline state, respectively. Dominant but less intense peaks were observed at 783 cm−1 which is assigned to the C–H out of plane wagging vibration of the benzenoid ring and at 652 cm−1 due to the quinoid deformation, which is probably with the interaction of chitosan with the PANI matrix or overlapping of the CS and PANI bands.37 The CS–SnO2–PANI hybrid composites bands at 1340 (C–C stretching vibrations of benzenoid) and 1556 cm−1 (C–N stretching vibrations of quinoid rings) are appeared, which revealed the presence of the oxidized PANI. An additional peak at 576 cm−1 was ascribed to stretching mode of the Sn–O bonds in the SnO2 rutile structure. Compared with the CS–PANI, the Raman spectrum of CS–SnO2–PANI shows the stretching modes were slightly shifted to higher wavenumber and decreased the relative intensity of the stretching vibration bands for the benzenoid and quinoid rings, which is attributed to hydrogen bond formation between SnCl4 and the polymer N–H groups into the Sn(OH)4 particle surface. The hybrid composites exhibited small Raman peak shifts due to the π–π* interactions of the polymeric matrix with the SnO2 surfaces, which may be a disordered structure of the CS–PANI matrix.
N stretching vibrations of the benzenoid and quinonoid units in the oxidized polyaniline state, respectively. Dominant but less intense peaks were observed at 783 cm−1 which is assigned to the C–H out of plane wagging vibration of the benzenoid ring and at 652 cm−1 due to the quinoid deformation, which is probably with the interaction of chitosan with the PANI matrix or overlapping of the CS and PANI bands.37 The CS–SnO2–PANI hybrid composites bands at 1340 (C–C stretching vibrations of benzenoid) and 1556 cm−1 (C–N stretching vibrations of quinoid rings) are appeared, which revealed the presence of the oxidized PANI. An additional peak at 576 cm−1 was ascribed to stretching mode of the Sn–O bonds in the SnO2 rutile structure. Compared with the CS–PANI, the Raman spectrum of CS–SnO2–PANI shows the stretching modes were slightly shifted to higher wavenumber and decreased the relative intensity of the stretching vibration bands for the benzenoid and quinoid rings, which is attributed to hydrogen bond formation between SnCl4 and the polymer N–H groups into the Sn(OH)4 particle surface. The hybrid composites exhibited small Raman peak shifts due to the π–π* interactions of the polymeric matrix with the SnO2 surfaces, which may be a disordered structure of the CS–PANI matrix.
3.3. Structural analysis
X-ray diffraction patterns of PANI, CS–SnO2, CS–PANI and CS–SnO2–PANI hybrid composites are shown in Fig. 2. The PANI diffractions were generally broad and less intense, which implies an amorphous crystalline nature. The diffraction broad band peak at 2θ = 20–30°, which is due to the periodicity parallel to the PANI matrix.38,39 The CS–SnO2 particle was observed with peaks at 2θ = 17.7° and 23.0° obtained that were normally broader and of lower intensity, which indicates a low degree of crystallinity for the biopolymer backbone chains, which implies a semi crystalline nature.40 The appearance of peaks for (110), (101), (002), (220), (301) and (202) confirmed a rutile structure for SnO2; the lattice parameter values were consistent with standard card values (JCPDS = 41-1445).41 The CS–PANI showed distinct crystalline peaks that were broad in nature due to the presence of chitosan (biopolymer) and PANI matrix.25 The CS–SnO2–PANI hybrid composite exhibited additional sharp and intense peaks responsible for SnO2 particles that were crystalline in nature. The CS–SnO2–PANI hybrid composite were more crystalline nature than CS–PANI and PANI. These results indicate that the crystalline structure of SnO2 had changed the CS–PANI composite structure. The phenomenon might be explained by the reason that SnO2 and coupling agent restricts the arrangement of molecular chains of the CS–PANI composite. These results are consistent with the HR-SEM analysis.
 |
| | Fig. 2 XRD patterns of (a) PANI, (b) CS–SnO2, (c) CS–PANI and (d) CS–SnO2–PANI ternary hybrid composites. | |
3.4. Morphological analysis
The surface morphologies of PANI, CS–SnO2, CS–PANI and CS–SnO2–PANI hybrid composites are shown in Fig. 3. The HR-SEM images of PANI showed an entangled layer-like structure42 and CS–SnO2 showed agglomerated particles in the surface morphology.43 The SEM image of CS–PANI (incorporation of biopolymer into the PANI matrix) showed the surface having a twisted fibrous structure with a semi-crystalline form. In a CS–SnO2–PANI hybrid composite, the individual small bright spots of CS–SnO2 particles were incorporated into the surface of the PANI matrix, which shows CS–SnO2 particles were homogeneously distributed with the chain surfaces of the PANI matrix. The Sn4+ doping with the multiple doping positions of the CS and PANI molecular chains, due to the increased number of nitrogen sites in the biopolymer and conducting polymer chains. Upon comparing the morphologies of the CS–PANI and CS–SnO2–PANI hybrid composites, a remarkable change was observed in the physical nature of the PANI and CS–SnO2. The elemental compositions of the hybrid composites obtained from EDAX patterns are shown in Fig. 4. The synthesized hybrid materials obtained some peaks at 1.0, 8.6 and 3.75 keV due to the presence of Sn with low content of other element peaks at 0.24, 0.52 and 2.75 keV due to the presence of carbon, oxygen and sulphur, respectively. The weight percent ratios obtained from Fig. 4 show the existence of impurities, which may have originated from the source materials or precipitating agent.25 It was clearly observed that the hybrid composite of chitosan and tin oxide were well incorporated into the polyaniline matrix, which also supports the FTIR and Raman results.
 |
| | Fig. 3 HR-SEM image of (a) PANI, (b) CS–SnO2, (c) CS–PANI and (d) CS–SnO2–PANI ternary hybrid composites. | |
 |
| | Fig. 4 Elemental analysis for (a) PANI, (b) CS–SnO2, (c) CS–PANI and (d) CS–SnO2–PANI ternary hybrid composites by EDXS. | |
The hybrid composite was also examined by FEG-TEM analysis, which is shown in Fig. 5. The TEM image of the composites shows that chitosan and SnO2 were uniformly distributed in the PANI matrix. The TEM image of PANI and CS–PANI (twist structure) composite was 100 nm.44 The CS–SnO2 composites show that had a very small size of SnO2 nanoparticle covered on the chitosan matrix. For the CS–SnO2–PANI hybrid composite, the SnO2 and chitosan were uniformly distributed over the PANI matrix with ca. 5.75 nm mean size (observed from the TEM image) that also prevents the chitosan matrix from aggregating. This indicates that the metal ions Sn4+ with multiple doping positions were combined with several hydroxyl and nitrogen sites in the chitosan and PANI chains, respectively. The crystal structure of SnO2 was confirmed by selected area electron diffraction (SAED) (inset in Fig. 5d), which showed four spotted rings that were consistent with the XRD patterns of different planes of the tetragonal SnO2 structure.45
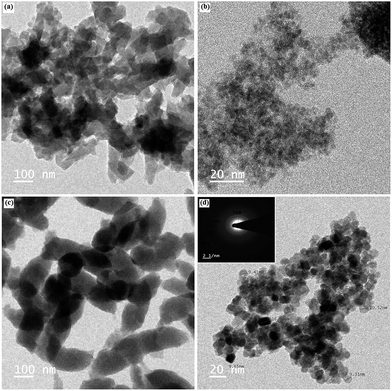 |
| | Fig. 5 FEG-TEM image of (a) PANI, (b) CS–SnO2, (c) CS–PANI and (d) CS–SnO2–PANI ternary hybrid composites. | |
3.5. Thermogravimetric analysis (TGA)
Thermograms of PANI, CS–SnO2, CS–PANI and CS–SnO2–PANI hybrid composites are illustrated in Fig. 6. The first weight loss of 10–20% observed at 100–120 °C was due to loss of water in all the polymeric composites. The second stage observed within the temperature range of 164–280 °C is attributed to removal of dopant molecules from the polymeric structure. The weight loss observed between 408 and 465 °C corresponded to the polymer chain degradation. The thermal stability of CS–PANI could be greatly increased by just a small addition of SnO2, and the thermal stability of CS–SnO2–PANI hybrid composites increased due to the SnO2 content. There was still 82.7%, 2.8% and 64.7% weights remaining in CS–SnO2, CS–PANI and CS–SnO2–PANI, respectively, when the temperature reached 800 °C.46 This should be attributed to the polymer and polysaccharides carbonization. It was demonstrated that the CS–SnO2–PANI ternary hybrid composite possessed higher thermal stability than CS–PANI.
 |
| | Fig. 6 TGA thermo grams of (a) PANI, (b) CS–SnO2, (c) CS–PANI and (d) CS–SnO2–PANI ternary hybrid composites. | |
3.6. BET analysis
The surface area and porosity property of the as-synthesized CS–PANI and CS–SnO2–PANI hybrid composite were further investigated by N2-adsorption/desorption behavior, as shown in Fig. 7. A typical IV isotherm with a H3-type hysteresis loops (P/P0 > 0.45) was observed, which indicated the presence of a mesoporous PANI matrix. In the high P/P0 region, such hysteresis loops did not exhibit any limiting adsorption, which is commonly attributed to particle aggregates with slit-shaped pores. The Brunauer–Emmett–Teller (BET) surface area of the hybrid composites materials was estimated to be 29.90 and 147.14 m2 g−1, respectively. The corresponding pore size distribution curve calculated from the desorption by the Barrett–Joyner–Halenda (BJH) method indicates aggregation of the hybrid composite and a relative wide pore size distribution in the range of 2.18–8.97 nm. The measured BET surface area for the formation of the twist fibrous structure CS–SnO2–PANI higher than synthesized material of CS–PANI, which may be due to the high content of SnO2 that would affect the CS–PANI growth. Thus the results demonstrate that the introduction of a SnO2 high content into the CS–PANI matrix can be effectively increase the surface area and is closely related to the particle size, which is beneficial for improving the electrochemical performance.47,48
 |
| | Fig. 7 N2 adsorption–desorption isotherm of (a) CS–PANI and (b) CS–SnO2–PANI ternary hybrid composites. | |
3.7. Cyclic voltammetry
To evaluate the electrochemical performance of the CS–PANI and CS–SnO2–PANI hybrid composites as active materials for modified glassy carbon electrodes, a platinum reference electrode and calomel counter electrode were carried out in a three electrode system. The electrochemical measurements were taken in an aqueous 1 M H2SO4 electrolyte solution in the potential range −0.2 to 1.0 V at the same time scan rate of 50 mV s−1 (Fig. 8a and b) shows the different scan rates between 10 and 200 mV s−1 that were measured in the same electrolyte. However, the electrode showed higher faradaic capacitance with clear redox peaks at all scan rates in the CV curves when measured in the H2SO4 electrolyte solution. These also exhibited apparent pseudo capacitance from CS, PANI and SnO2 particles.49 The specific capacitance of the composite materials can be calculated according to the following equations from the CV curves as follows:| |
 | (1) |
where (Q) is the total voltammetric charge, (V) is the scan rate and (m) is the mass of the composite electrode material. This shows the modified electrode materials combined with two different energy storage mechanisms was calculated simultaneously, for measuring electrical double layer capacitance (EDLC) and faradaic capacitance.50 The modified glassy carbon electrode showed redox peaks that were the characteristic of pseudo-capacitance with the specific capacitance of CS–PANI and CS–SnO2–PANI calculated to be 84.34 and 179.20 F g−1 at 10 mV s−1, respectively. It can be concluded that the modified electrode would have potential applications in supercapacitor materials. The specific capacitance values obtained for as prepared CS–SnO2–PANI hybrid composite electrode showed higher values than the values of previous published study (Table 1).
 |
| | Fig. 8 Cyclic voltammograms of CS–PANI and CS–SnO2–PANI ternary hybrid composites (a) scan rate of 50 mV s−1 in 1 M H2SO4; (b and c) different scan rates (10–200 mV s−1); (d) specific capacitance calculated by the CV curve. | |
Table 1 Specific capacitance comparison of the ternary hybrid composite electrode with previously reported study
| S. No. |
Material |
Electrolyte |
Current density |
Specific capacitance |
Reference |
| 1 |
LiCo4 doped chitosan/starch blend electrolyte |
— |
CV scan 10 mV s−1 |
133 F g−1 |
29 |
| 2 |
Chitosan based gel electrolyte |
— |
2.5 mA g−1 |
131 F g−1 |
30 |
| 3 |
Graphene–SnO2/PEDOT |
1 M H2SO4 |
CV scan 10 mV s−1 |
184 F g−1 |
31 |
| 4 |
CNO–PANI01 |
1 M H2SO4 |
CV scan 10 mV s−1 |
33 F g−1 |
32 |
| 5 |
Chitosan–SnO2–PANI |
1 M H2SO4 |
CV scan 10 mV s−1 |
179.2 F g−1 |
This work |
The specific capacitance is an important parameter to evaluate supercapacitor performance; Fig. 9a–c, shows the first charge–discharge curves of the CS–PANI and CS–SnO2–PANI hybrid composite electrodes in 1.0 M H2SO4 solution at different current densities (0.1, 0.5 and 1 A g−1) within the potential window of −0.2 to 0.8 V. There is an obvious deviation of the discharge curves from a straight line, which demonstrated that the capacitance mainly comes from the faradaic redox reaction of the CS–PANI and CS–SnO2–PANI hybrid composite electrode displayed energy (E) and power density (P), as indicated by the following equations:51
| | |
E = 1000 × Cs(ΔV)2/2 × 3600
| (2) |
 |
| | Fig. 9 (a) Charge–discharge curve of CS–PANI and CS–SnO2–PANI ternary hybrid composites, (b) CS–PANI and (c) CS–SnO2–PANI in charge–discharge curves at different current densities (0.1, 0.5 and 1.0 A g−1). | |
Electrochemical performance of hybrid composite electrode for specific capacitance, energy density and power density values was given in the Table 2. From the table, it is observed that CS–SnO2–PANI hybrid composite electrode material could be attributed to the excellent capacitive performance.
Table 2 Electrochemical performance of CS–PANI and CS–SnO2–PANI hybrid composite electrode
| S. No. |
Samples |
Specific capacitance (F g−1) |
Energy density (W h kg−1) |
Power density (kW kg−1) |
| 1 |
CS–PANI |
84.34 |
4.21 |
15.18 |
| 2 |
CS–SnO2–PANI |
179.20 |
8.96 |
32.25 |
3.8. Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy (EIS) was used to analyze the modified electrodes electrochemical behavior at the electrode/electrolyte interface. The ideal Nyquist impedance plot was composed of a half semicircle at high frequency and a line on the low frequency side. Fig. 10 shows the AC impedance spectra of CS–PANI and CS–SnO2–PANI modified electrodes measured in 1 M H2SO4 electrolyte under a 5 mV s−1 potential amplitude and frequency range of 105–0.01 Hz. The experimental results were fitted using an appropriate equivalent circuit. In the high frequency region, a small arc related to the process at the electrode-materials electrolyte interface was observed, which is normally expected to be the capacitance in parallel with the ionic charge-transfer resistance (Rct). At the lower frequency the electrode tested in H2SO4 showed 90° straight lines, showing typical capacitance behavior.45,49,52 These results, when the electrode tested in H2SO4 electrolyte solution had a lower CS–SnO2–PANI modified electrode charge-transfer resistance may be due to the immobilization effect of chitosan, SnO2 and PANI, in which the twisted fibrous structure made it possible to efficiently access and move electrolyte ions to the modified electrode surface and shorten the ion-diffusion path. Thus, better capacitive behavior for CS–SnO2–PANI modified electrode over CS–PANI was observed.
 |
| | Fig. 10 Electrochemical impedance spectra of (a) CS–PANI and (b) CS–SnO2–PANI ternary hybrid composites. | |
4. Conclusion
A new ternary hybrid composite of chitosan, SnO2 and PANI were successfully synthesized by a simple oxidative chemical precipitation method. The synthesized hybrid composite was characterized by FTIR, Raman, XRD, HR-SEM with EDAX, TEM, TGA and BET, which revealed that CS–SnO2 was uniformly mixed within the PANI matrix. The CS–SnO2–PANI modified electrode exhibited outstanding electrode performance with a high specific capacitance, such as a 179.20 F g−1 specific capacitance value achieved in 1 M H2SO4 solution at 10 mV s−1 with a maximum 8.96 (W h kg−1) energy density and 32.25 (kW kg−1) power density for the electrochemical capacitors performance using CV, charge–discharge cycling and impedance spectroscopy. This study reports potential electroactive materials for the application of the next generation super capacitors.
Acknowledgements
The authors greatly acknowledge the Department of Industrial Chemistry for providing the HR-SEM facility and the Department of Physics for the XRD characterization, Alagappa University, Karaikudi.
References
- J. Yan, T. Wei, B. Shao, Z. Fan, W. Qian, M. Zhang and F. Wei, Carbon, 2010, 48, 487–493 CrossRef CAS.
- A. Zhang, L. Wang, L. Zhang and Y. Zhang, J. Appl. Polym. Sci., 2010, 115, 1881–1885 CrossRef CAS.
- N. Kumar and J. Baek, Chem. Commun., 2014, 50, 6298–6308 RSC.
- H. Wang, Q. Hao, X. Yang, L. Lu and X. Wang, Nanoscale, 2010, 2, 2164–2170 RSC.
- F. Chen, P. Liu and Q. Zhao, Electrochim. Acta, 2012, 76, 62–68 CrossRef CAS.
- U. Lange, N. V. Roznyatovskaya and V. M. Mirsky, Anal. Chim. Acta, 2008, 614, 1–26 CrossRef CAS PubMed.
- J. Wang, B. Deng, H. Chen, X. Wang and J. Zheng, Environ. Sci. Technol., 2009, 43, 5223–5228 CrossRef CAS PubMed.
- D. D. Potphode, P. Sivaraman, S. P. Mishra and M. Patri, Electrochim. Acta, 2015, 155, 402–410 CrossRef CAS.
- M. S. Cho, S. Y. Park, J. Y. Hwang and H. J. Choi, Mater. Sci. Eng., C, 2004, 24, 15–18 CrossRef.
- R. B. Rakhi, W. Chen and H. N. Alshareef, J. Mater. Chem., 2012, 22, 5177–5183 RSC.
- S. Anandhavelu and S. Thambidurai, Ionics, 2013, 19, 903–909 CrossRef CAS.
- Z. Sun, Z. Bai, H. Shen, S. Zheng and R. L. Frost, Mater. Res. Bull., 2013, 48, 1013–1019 CrossRef CAS.
- J. Jouhannaud, J. Rossignol and D. Stuerga, J. Solid State Chem., 2008, 181, 1439–1444 CrossRef CAS.
- S. Anandhavelu and S. Thambidurai, Carbohydr. Polym., 2011, 83, 1565–1569 CrossRef CAS.
- Z. Zainal, L. K. Hue, M. Z. O. Hussein, A. H. Abdullah and I. R. Hamadneh, J. Hazard. Mater., 2009, 164, 138–145 CrossRef CAS PubMed.
- J. Y. Chen, P. J. Zhou, J. L. Li and Y. Wang, Carbohydr. Polym., 2008, 72, 128–132 CrossRef CAS.
- A. A. Ansari, A. Kaushik, P. R. Solanki and B. D. Malhotra, Electroanalysis, 2009, 21, 965–972 CrossRef CAS.
- H. Zhu, R. Jiang, L. Xiao, Y. Chang, Y. Guan, X. Li and G. Zeng, J. Hazard. Mater., 2009, 169, 933–940 CrossRef CAS PubMed.
- A. G. Yavuz, A. Uygun and V. R. Bhethanabolta, Carbohydr. Polym., 2009, 75, 448–453 CrossRef CAS.
- J. Chen, Z. Xia, H. Li, Q. Li and Y. Zhang, Electrochim. Acta, 2015, 166, 174–182 CrossRef CAS.
- X. M. He, G. T. Zhu, H. B. Zheng, S. N. Xu, B. F. Yuan and Y. Q. Feng, Talanta, 2015, 140, 29–35 CrossRef CAS PubMed.
- S. B. Kondawar, S. A. Acharya and S. R. Dhakate, Adv. Mater. Lett., 2011, 2, 362–367 CAS.
- S. B. Kondawar, S. P. Agrawal, S. H. Nimkar and H. J. Sharma, Adv. Mater. Lett., 2012, 3, 393–398 CAS.
- H. Y. Zhu, L. Xiao, R. Jiang, G. M. Zeng and L. Liu, Chem. Eng. J., 2011, 172, 746–753 CrossRef CAS.
- K. Pandiselvi and S. Thambidurai, Polym. Degrad. Stab., 2013, 1–9 Search PubMed.
- S. Zavareh, M. Zarei, F. Darvishi and H. Azizi, Chem. Eng. J., 2015, 273, 610–621 CrossRef CAS.
- H. Xu, X. Chen, J. Zhang, J. Wang, B. Cao and D. Cui, Sens. Actuators, B, 2013, 176, 166–173 CrossRef CAS.
- S. Selvam, B. Balamuralitharan, S. N. Karthick, A. Dennyson Savariraj, K. V. Hemalatha, S.-K. Kim and H.-J. Kim, J. Mater. Chem. A, 2015, 3, 10225–10232 CAS.
- F. Kadi Allah, S. Yapi Abe, C. M. Nunez, A. Khelil, L. Cattin, M. Morsli, J. C. Bernede, A. Bougrine, M. A. Del Valle and F. R. Diaz, Appl. Surf. Sci., 2007, 253, 9241–9247 CrossRef.
- S. Ameen, M. S. Akhtar, Y. S. Kim, O. B. Yang and H. S. Shin, Microchim. Acta, 2011, 172, 471–478 CrossRef CAS.
- W. Wenjuan, L. Wu, Y. Tianyuan, X. Xifeng, H. Wenjuan, H. Qingli and W. Xin, Electrochim. Acta, 2013, 10, 118–126 Search PubMed.
- U. Susmitha, M. Umasankar and S. Palaniappan, Electrochim. Acta, 2014, 146, 242–248 CrossRef.
- D. Acevedo, C. Rivarola, M. Miras and C. Barbero, Electrochim. Acta, 2011, 56, 3468–3473 CrossRef CAS.
- J. Gajendiran and V. Rajendran, Mater. Lett., 2015, 139, 116–118 CrossRef CAS.
- C. Valle's, P. Jimenez, E. Munoz, A. M. Benito and W. K. Maser, J. Phys. Chem. C, 2011, 115, 10468–10474 Search PubMed.
- L. Zheng, C. Chen, Y. Zheng, Y. Zhan, Y. Cao, X. Lin, Q. Zheng, K. Wei and J. Zhu, Appl. Catal., B, 2014, 149, 44–50 CrossRef.
- K. Pandiselvi and S. Thambidurai, Int. J. Biol. Macromol., 2014, 67, 270–278 CrossRef CAS PubMed.
- D. K. Bandgar, S. T. Navale, A. T. Mane, S. K. Gupta, D. K. Aswal and V. B. Patil, Synth. Met., 2015, 204, 1–9 CrossRef CAS.
- A. T. Ramaprasad, V. Rao, G. Sanjeev, S. P. Ramanani and S. Sabharwal, Synth. Met., 2009, 159, 1983–1990 CrossRef CAS.
- S. Anandhavelu and S. Thambidurai, Mater. Chem. Phys., 2011, 131, 449–454 CrossRef CAS.
- M. Abdullah, A. Hamdi, M. Sillanpaa and J. Dutta, J. Alloys Compd., 2015, 618, 366–371 CrossRef.
- A. N. Naveen and S. Selladurai, Mater. Sci. Semicond. Process., 2015, 40, 468–478 CrossRef.
- K. Yun, C. Guobing, L. Xiaodong, L. Liang, S. Xiaoming and K. Yun, Sens. Actuators, B, 2013, 81, 629–636 Search PubMed.
- M. R. Nabid, M. Golbabaee, A. B. Moghaddam, R. Dinarvan and R. Sedghi, Int. J. Electrochem. Sci., 2008, 3, 1117–1126 CAS.
- Z. Wu, X. Li, L. Tai, H. Song, Y. Zhang, B. Yan, L. Fan, H. Shan and D. Li, J. Alloys Compd., 2015, 646, 1009–1014 CrossRef CAS.
- J. Wang, Z. Wu, K. Hu, X. Chen and H. Yin, J. Alloys Compd., 2015, 619, 38–43 CrossRef CAS.
- K. Pandiselvi and S. Thambidurai, Colloids Surf., B, 2013, 108, 229–238 CrossRef PubMed.
- Y. Masuda, Prog. Cryst. Growth Charact. Mater., 2012, 58, 106–120 CrossRef CAS.
- Y. Jin and M. Jia, Colloids Surf., A, 2015, 464, 17–25 CrossRef CAS.
- V. H. Nguyen and J.-J. Shima, Synth. Met., 2015, 207, 110–115 CrossRef CAS.
- E. Raphael Ezeigwe, M. T. T. Tan, P. S. Khiew and C. W. Siong, Ceram. Int., 2015, 41, 715–724 CrossRef.
- K. Pandiselvi and S. Thambidurai, Ionics, 2014, 20, 551–561 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching modes of the quinoid and benzenoid rings. The peaks at 1299, 1118 and 804 cm−1 are attributed to the stretching vibrations of benzenoid rings (C–N, C
C stretching modes of the quinoid and benzenoid rings. The peaks at 1299, 1118 and 804 cm−1 are attributed to the stretching vibrations of benzenoid rings (C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–C, respectively).33 The CS–SnO2 composite had obtained two strong peaks at 658 and 567 cm−1 that are ascribed to the Sn–O–Sn vibration in the SnO2 particles. The main characteristic chitosan functional group was observed at 1633 cm−1 due to the bending vibration of –NH2 group in the glucopyranose ring of chitosan.34 The location of the CS–PANI main characteristic peaks are in good agreement with the literature.17,19 This broadening of the absorbed bands between 2918 and 3428 cm−1 was due to the H-bonding interaction between chitosan and PANI. The characteristic bands at 1565 and 1485 cm−1 are the C
N and C–C, respectively).33 The CS–SnO2 composite had obtained two strong peaks at 658 and 567 cm−1 that are ascribed to the Sn–O–Sn vibration in the SnO2 particles. The main characteristic chitosan functional group was observed at 1633 cm−1 due to the bending vibration of –NH2 group in the glucopyranose ring of chitosan.34 The location of the CS–PANI main characteristic peaks are in good agreement with the literature.17,19 This broadening of the absorbed bands between 2918 and 3428 cm−1 was due to the H-bonding interaction between chitosan and PANI. The characteristic bands at 1565 and 1485 cm−1 are the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration of the quinoid and benzenoid rings, respectively. The band at 1301 cm−1 is the aromatic C–N stretching vibration, 1112 cm−1 is assigned to the N
C stretching vibration of the quinoid and benzenoid rings, respectively. The band at 1301 cm−1 is the aromatic C–N stretching vibration, 1112 cm−1 is assigned to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration and 810 cm−1 is the aromatic C–H out of plane bending vibration.27 The CS–SnO2–PANI hybrid composites show that all characteristic bands of CS–PANI appeared between 400 and 1600 cm−1 region and these were all found in the hybrid composites, although the relative intensity of some bands had shifted into higher wavenumber compared to CS–PANI due to the presence of SnO2. This shift may be ascribed to the formation of hydrogen bonding between the SnCl4 and N–H group of CS–PANI on the surface of the Sn(OH)4 particles.25 It is interesting to note that adding SnCl4 reduced the intensity of the quinine and benzene bands to be much weaker than that in CS–PANI and a new strong absorption peak at 501 cm−1 was attributed to the Sn–O–Sn bond vibrations of the Sn–O–Sn bonds in SnO2. This indicates that amine and imine (N–H and N
N vibration and 810 cm−1 is the aromatic C–H out of plane bending vibration.27 The CS–SnO2–PANI hybrid composites show that all characteristic bands of CS–PANI appeared between 400 and 1600 cm−1 region and these were all found in the hybrid composites, although the relative intensity of some bands had shifted into higher wavenumber compared to CS–PANI due to the presence of SnO2. This shift may be ascribed to the formation of hydrogen bonding between the SnCl4 and N–H group of CS–PANI on the surface of the Sn(OH)4 particles.25 It is interesting to note that adding SnCl4 reduced the intensity of the quinine and benzene bands to be much weaker than that in CS–PANI and a new strong absorption peak at 501 cm−1 was attributed to the Sn–O–Sn bond vibrations of the Sn–O–Sn bonds in SnO2. This indicates that amine and imine (N–H and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) group) nitrogen atoms bonded with Sn4+ via either protonation or coordination complexation. Thus, results indicate the interfacial interaction between CS–PANI and the inorganic SnO2 particle counterpart of the SnO2 particles.
group) nitrogen atoms bonded with Sn4+ via either protonation or coordination complexation. Thus, results indicate the interfacial interaction between CS–PANI and the inorganic SnO2 particle counterpart of the SnO2 particles.

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) stretching vibrations of the benzenoid rings and at 1556 cm−1 are attributed to C
C) stretching vibrations of the benzenoid rings and at 1556 cm−1 are attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations of the quinonoid units.35 The CS–SnO2 had a band around 576 cm−1 that is ascribed to Sn–O stretching mode and the peak centered at 771 cm−1 can be assigned to the SnO2 mode, which are related to the expansion and contraction vibrational modes of the Sn–O bonds in rutile SnO2.36 The CS–PANI spectrum shows representative peaks at 1340 and 1556 cm−1, corresponding to the C
N stretching vibrations of the quinonoid units.35 The CS–SnO2 had a band around 576 cm−1 that is ascribed to Sn–O stretching mode and the peak centered at 771 cm−1 can be assigned to the SnO2 mode, which are related to the expansion and contraction vibrational modes of the Sn–O bonds in rutile SnO2.36 The CS–PANI spectrum shows representative peaks at 1340 and 1556 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations of the benzenoid and quinonoid units in the oxidized polyaniline state, respectively. Dominant but less intense peaks were observed at 783 cm−1 which is assigned to the C–H out of plane wagging vibration of the benzenoid ring and at 652 cm−1 due to the quinoid deformation, which is probably with the interaction of chitosan with the PANI matrix or overlapping of the CS and PANI bands.37 The CS–SnO2–PANI hybrid composites bands at 1340 (C–C stretching vibrations of benzenoid) and 1556 cm−1 (C–N stretching vibrations of quinoid rings) are appeared, which revealed the presence of the oxidized PANI. An additional peak at 576 cm−1 was ascribed to stretching mode of the Sn–O bonds in the SnO2 rutile structure. Compared with the CS–PANI, the Raman spectrum of CS–SnO2–PANI shows the stretching modes were slightly shifted to higher wavenumber and decreased the relative intensity of the stretching vibration bands for the benzenoid and quinoid rings, which is attributed to hydrogen bond formation between SnCl4 and the polymer N–H groups into the Sn(OH)4 particle surface. The hybrid composites exhibited small Raman peak shifts due to the π–π* interactions of the polymeric matrix with the SnO2 surfaces, which may be a disordered structure of the CS–PANI matrix.
N stretching vibrations of the benzenoid and quinonoid units in the oxidized polyaniline state, respectively. Dominant but less intense peaks were observed at 783 cm−1 which is assigned to the C–H out of plane wagging vibration of the benzenoid ring and at 652 cm−1 due to the quinoid deformation, which is probably with the interaction of chitosan with the PANI matrix or overlapping of the CS and PANI bands.37 The CS–SnO2–PANI hybrid composites bands at 1340 (C–C stretching vibrations of benzenoid) and 1556 cm−1 (C–N stretching vibrations of quinoid rings) are appeared, which revealed the presence of the oxidized PANI. An additional peak at 576 cm−1 was ascribed to stretching mode of the Sn–O bonds in the SnO2 rutile structure. Compared with the CS–PANI, the Raman spectrum of CS–SnO2–PANI shows the stretching modes were slightly shifted to higher wavenumber and decreased the relative intensity of the stretching vibration bands for the benzenoid and quinoid rings, which is attributed to hydrogen bond formation between SnCl4 and the polymer N–H groups into the Sn(OH)4 particle surface. The hybrid composites exhibited small Raman peak shifts due to the π–π* interactions of the polymeric matrix with the SnO2 surfaces, which may be a disordered structure of the CS–PANI matrix.











