All solid supercapacitors based on an anion conducting polymer electrolyte†
Chenxi Xua,
Jian Yan*ab,
Qingqing Qinc,
Yuming Denga,
Jigui Chenga,
Yong Zhangab and
Yucheng Wu*ab
aSchool of Materials Science and Engineering, Hefei University of Technology, Hefei, 230009, China. E-mail: yanjian@hfut.edu.cn; ycwu@hfut.edu.cn
bKey Laboratory of Advanced Functional Materials and Devices of Anhui Province, Hefei, 230009, China
cInstitute of Industry & Equipment Technology, Hefei University of Technology, Hefei, 230009, China
First published on 9th February 2016
Abstract
In this paper, KOH doped polybenzimidazole (PBI–KOH), an anion conducting polymer electrolyte, has been employed to demonstrate the possibility of fabricating alkaline all solid supercapacitors. This is different from the conventional gel-electrolyte based quasi-solid supercapacitors. Symmetric devices assembled using commercial activated carbon based electrodes and PBI–KOH electrolytes exhibit a high rate capability with specific capacitance retention above 90% when the discharge current density increased from 0.5 to 20 A g−1. However, PBI–KOH suffers from low cycling stability with a rapid decrease in specific capacitance in the first 1000 cycles. By adding a binder or narrowing the potential window, the cycling stability could be improved. The best device shows a quite stable performance during the first 2000 cycles with specific capacitance degradation of less than 5%. An asymmetric (or hybrid) device has been assembled using activated carbon and Ni(OH)2 as electrode materials, which exhibits a relative high specific energy of 37.1 W h kg−1. These results strongly recommend the great potential of solid anion conducting polymer electrolytes in developing alkaline all solid supercapacitors.
1. Introduction
Supercapacitors with higher power density, long cycle life and moderate energy density have attracted significant attention for practical applications.1–5 In very recent years, the fast increasing demands in wearable electronic devices make flexible (and/or stretchable) supercapacitors one of the hottest research topics.6–10 These flexible supercapacitors are commonly assembled using polyvinyl alcohol (PVA) based gel electrolytes like PVA–KOH, PVA–H2SO4 or PVA–H3PO4 gel electrolytes, featuring advantages of low cost, high water absorbing and holding capacity, which are suitable for fabricating flexible quasi-solid supercapacitors.11–14 However, they still suffer from evaporation of solvent, which may cause safety issues and loss of ion conductivity. Solid polymer electrolytes exhibit superiorities including easy handling without spillage of liquid electrolyte, higher safety and more flexibility in packaging etc., which have been proposed as superior candidates for fabricating true all solid supercapacitors (ASSs).15–17 As one of the examples, phosphoric acid doped polybenzimidazole (PBI) is a good proton conducting polymer.15,16 In addition, it shows better mechanical strength and dimensional stability which could lower the requirement of conductive substrate for flexible supercapacitors.17,18In 2012, Samui's group reported an ASS based on activated carbon (AC) and poly[2,5 benzimidazole] exhibiting a specific capacitance of 248 F g−1 at a high working temperature of 120 °C.16 They also fabricated an ASS based on polyaniline and crosslinked sulfonated poly[ether ether ketone] with a high specific capacitance of 480 F g−1.19 Pillai and co-workers have fabricated an ASS using RuO2/carbon composites and phosphoric acid doped PBI, which shows a relative small equivalent series resistance (ESR) of about 3.7 Ω.15 Nafion based proton conducting polymers and sulfonated hydrocarbon-based proton conductors including poly(ether sulfones) and poly(ether ketones) with varying numbers of ether and ketone functionalities like poly(ether ether ketone) have also been widely used for electrochemical double layer capacitors and pseudo-capacitors.17 However, further improvement of the energy density of ASSs based on proton conducting polymer electrolytes remain a challenge due to the lack of suitable negative electrode materials for RuO2 and PANI based positive electrode materials.17,20,21 Alkaline electrolytes based supercapacitor electrode materials provide a large potential window,4,12 e.g. asymmetric device assembled with AC and Ni(OH)2 (or NiO) could stand up to 1.5 V or higher.8,13 Therefore, it would be easier to achieve higher energy density since Ni and other alkaline based electrode materials also exhibit high specific capacitance.22,23 Meanwhile, in recent years, anion exchange membranes (AEMs) have been also widely investigated for the applications in fuel cells.24–26 As one of the AEMs, PBI exhibits superior anion conductivity when doped with KOH.27 It suggests that PBI doped with KOH (PBI–KOH) could serve as anion conducting polymer electrolyte for fabricating alkaline ASSs with the attempt to achieve high energy density.
Inspired by these studies, we have assembled ASSs using commercial available AC as active materials and PBI–KOH electrolyte. The assembled devices exhibit lower ESR and better performance when doped with higher concentration KOH. A relative high specific capacitance of 40 F g−1 (based on total mass of AC in two electrodes) was achieved in a potential window of 0–1 V. However, the cycling stability is low when PBI soaked in higher concentration KOH. Shrinking the potential window and adding poly(vinylidene fluoride) (PVDF) are two possible ways to improve the stability. At a potential window from −0.5 V to +0.5 V, the device could endure 5500 cycles test with specific capacitance remaining 70% of its highest one. By mixing PVDF with AC in the electrodes, the specific capacitance degradation is below 5% after 2000 cycles indicating good cycling stability. More importantly, an asymmetric device has been assembled using AC and Ni(OH)2 as active materials and PBI–KOH as electrolyte. A highest specific capacitance of 118.6 F g−1 (specific energy: 37.1 W h kg−1) was achieved. The current work doubtlessly demonstrates that PBI–KOH is a promising candidate in developing high energy density ASSs.
2. Experimental
2.1 Materials
AC (JCAC-2000, specific surface area: 2000 m2 g−1) were purchased from Nanjing JCNANO Tech Co. Ltd. PBI powders were purchased from Shanghai Shengjun Plastics Technologies Co. Ltd. All the chemicals were used without further purifying.2.2 Preparation of PBI film and Ni(OH)2 spheres
PBI films were prepared by a drop casting method using PBI dissolved in dimethylacetamide (DMAC) (5 wt%). The thickness of films were tuned from 10 (±25%) to 40 (±25%) μm. Then the PBI films were soaked in KOH solution with different concentrations for certain period.The Ni(OH)2 nanospheres were synthesized by a simple precipitation method. Typically, NiSO4 2 mmol was dissolved in 50 ml deionized-water (DI-water). In the following, 2 mmol NH3·H2O has been added to the solution drop by drop with stirring at 65 °C heated by a hotplate for 30 min. The mixture was then centrifuged. The production was collected and dried at 60 °C overnight.
2.3 Characterization
The phase of the product was identified by X-ray powder diffraction (XRD, X'Pert PRO MPD), using Cu Kα (λ = 0.15406 nm) radiation at 40 kV and 40 mA in a 2θ range from 10° to 80° at room temperature. The morphology and structure of the products were characterized by field emission scanning electron microscopy (FESEM, SU8020) and with an X-ray energy dispersive spectrometer (EDS).2.4 Electrochemical measurement
Electrodes for three-electrodes test were prepared by mixing active materials (AC or Ni(OH)2 spheres), Super-P and PVDF with a mass ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in DMAC and N-methyl-2-pyrrolidone with volume ratio of 30
5 in DMAC and N-methyl-2-pyrrolidone with volume ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70. The mixture was stirred over night at 70 °C on hotplate and then coated on graphite paper or nickel foam with an area of 1 cm2 followed by drying at 100 °C for 12 h. After mixing active materials (AC or Ni(OH)2 spheres), Super-P (SP), PBI with or without PVDF with different mass ratios, electrodes for devices were prepared following above procedure. The mass of the active materials is obtained by measuring the weight difference of the electrode before and after coating process using a microbalance with an accuracy of 0.01 mg. The electrodes (AC//AC or AC//Ni(OH)2) were soaked with KOH solution for about 20 min. After that, PBI–KOH separator was put in between the two electrodes and pressed under pressure. The devices were not sealed and then clamped between two pieces of glass by clips.
70. The mixture was stirred over night at 70 °C on hotplate and then coated on graphite paper or nickel foam with an area of 1 cm2 followed by drying at 100 °C for 12 h. After mixing active materials (AC or Ni(OH)2 spheres), Super-P (SP), PBI with or without PVDF with different mass ratios, electrodes for devices were prepared following above procedure. The mass of the active materials is obtained by measuring the weight difference of the electrode before and after coating process using a microbalance with an accuracy of 0.01 mg. The electrodes (AC//AC or AC//Ni(OH)2) were soaked with KOH solution for about 20 min. After that, PBI–KOH separator was put in between the two electrodes and pressed under pressure. The devices were not sealed and then clamped between two pieces of glass by clips.
Electrochemical measurements of three electrodes system were carried out by an electrochemical analyzer (Autolab Potentiostat, PGSTAT101). The three-electrode cell consisted of Ag/AgCl as the reference electrode, Pt as the counter electrode and the as-prepared sample as the working electrode. 1 M KOH solution was served as electrolyte at room temperature. Cyclic voltammetry (CV) was done at different scan rates of 5, 10, 20, 50 and 100 mV s−1. Galvanostatic charge/discharge (CD) curves were measured at different current densities. As to the test of devices, CV and CD curves were tested by Autolab Potentiostat, PGSTAT101, while cycling performance measurement was performed by LAND BT2013S supercapacitor test system. The detailed information of the tested devices is listed in Table 1. It includes loading mass, mass ratio of the materials in electrode, concentration of KOH and the time for the soaking process of PBI films as separator with thickness provided and the concentration of KOH for the electrode soaking process.
| Device | Mass | AC | PBI | Super-P | PVDF | PBI thickness | KOH for PBI separator | Time | KOH for electrode |
|---|---|---|---|---|---|---|---|---|---|
| P1 | 3.3 mg | 14 | 3 | 3 | 30 μm | 1 M | 3 h | 1 M | |
| P2 | 2.5 mg | 14 | 3 | 3 | 30 μm | 1 M | 16 h | 2 M | |
| P3 | 2.3 mg | 14 | 3 | 3 | 30 μm | 1 M | 16 h | 6 M | |
| P4 | 2.3 mg | 14 | 3 | 3 | 18 μm | 2 M | 16 h | 6 M | |
| C1 | 2.3 mg | 14 | 3 | 3 | 35 μm | 4 M | 36 h | 4 M | |
| C2 | 4.6 mg | 14 | 3 | 3 | 12 μm | 1 M | 24 h | 1 M | |
| C3 | 1.3 mg | 14 | 3 | 3 | 40 μm | 1 M | 24 h | 1 M | |
| C4 | 2.3 mg | 14 | 3 | 3 | 22 μm | 1 M | 288 h | 1 M | |
| C5 | 2.0 mg | 50 | 30 | 15 | 5 | 30 μm | 6 M | 48 h | 6 M |
| C6 | 2.0 mg | 16 | 4 | 3 | 1 | 30 μm | 6 M | 48 h | 6 M |
| C7 | 2.8 mg | 16 | 4 | 3 | 1 | 30 μm | 6 M | 48 h | 6 M |
| C8 | 2.8 mg | 16 | 4 | 3 | 1 | 30 μm | 6 M | 48 h | 6 M |
| C9 | 1.6 mg | 14 | 3 | 3 | 12 μm | 1 M | 5 h | 1 M | |
| C10 | 2.1 mg | 14 | 3 | 3 | 25 μm | 1 M | 6 h | 1 M |
3. Results and discussions
3.1 Materials and structure characterization
Fig. 1a shows the typical XRD patterns of the as-prepared Ni-based precipitation. The weak and very broad XRD peaks indicate the poor crystalline nature. These peaks can be roughly indexed to the hexagonal β-Ni(OH)2 structure with lattice constants of a = 3.127 and c = 4.606 (JCPDS card no. 14-0117).28 SEM images of as-synthesized products are shown in Fig. 1b and c, which depict the uniform spheres like morphology of Ni(OH)2. The FE-SEM image with high magnification shows that the diameter of the Ni(OH)2 spheres are about several hundred nanometers. The spheres also exhibit rough surface. Elemental analysis by EDS reveals the presence of Ni and O (see Fig. S1†).According to Sun's report,25 there are two ways for combinations between PBI and KOH, and the possibility of K+ bonding to N− is much greater than that of K+ and –NH– due to the lower bond energy. However, the OH− could be the main role as exterior to help and make K+ displace H+ in –N–H with the H2O generation, while K+ was introduced into the polymer connecting with OH− to form a charge balance. The amine groups in PBI may act as proton donors to react with strong alkali of KOH, and also the water was adsorbed by PBI membrane during alkali doping. Some of KOH molecules probably were taken into the polymer by water molecules. The ion was hopping through the PBI matrix which provides the suitable ionic conductivity. The possible microstructure is shown in Scheme 1.
3.2 Electrochemical analysis
 | (1) |
These ASSs were further tested by the constant current charge/discharge method. Fig. 2c shows the specific capacitance at various current densities, which is calculated based on the following formula,
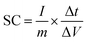 | (2) |
As expected, when thinner PBI separator (18 μm) is soaked with KOH at higher concentration (2 M), the resistance of device (P4) could be further reduced as shown in Fig. 2d. The specific capacitance degradation of device P4 is less than 5% even at a high discharge current density of 20 A g−1, suggesting a good rate capability. Since PBI–KOH serves as the ionic conductor, the ions transport is different from that in liquid electrolyte which is confined by the Fick's laws of diffusion.24,30,31 This might also be responsible for the good rate performance. However, irreversible reactions are observed at a low current density of 0.2 A g−1 during the charge process as revealed in Fig. 2e and f. The irreversible reactions are more severe when PBI is doped in KOH with higher concentration, which might cause the cycling stability issues. Although the content of PBI in the electrode may also affect the device performance (Fig. S2†), following discussion will be mainly on the long term cycling stability since it is the major obstacle of PBI–KOH electrolyte.27
When using low concentration KOH (1 M), the devices (C2 & C3) show better cycling performance as shown in Fig. 3c. The device (C3) keeps relatively stable in the first 2000 cycles while the specific capacitance decreases quickly in the following thousands cycles. Meanwhile, the device (C2) exhibits different degradation trend. Following the sharp reduction, the specific capacitance tends to be relatively stable. These results suggest that probably different degradation mechanisms worked in the reactions. When the PBI film is soaked in 1 M KOH for much longer time (288 h), the stability (device C4) becomes worse as revealed in Fig. 3d. Initially, the charge specific capacitance is much higher than discharge specific capacitance as there are serious irreversible reactions. The charge–discharge curves (Fig. S4†) depict that these irreversible reactions occur at relatively high voltage range during charge process. But, no irreversible reactions were found in the discharge process. Such irreversible reactions might accelerate the degradation of ionic conductivity of PBI–KOH.
To improve the cycling stability, PVDF was added as binder. During the first test of device C5, the specific capacitance is quite stable as shown in Fig. 4a. In the second test, however, the specific capacitance decreased much fast after irreversible reactions as indicated by the boxes. During the cycling test it may endure sever irreversible reactions depicted in Fig. 4b. It is unclear what caused such irreversible reactions, which might induce the degradation of ionic conductivity of PBI–KOH. Although the capacitance loss of the devices of C7 and C8 is also high (Fig. 4c and d), it exhibits slightly better performance than that of devices without binder (C1–C4). The capacitance retention of device C8 remains about 74% after 5000 cycles with the concentration of KOH at 6 M.
The irreversible reactions normally occur at relatively high voltage range (see Fig. S4†). Therefore, it is possible to suppress such irreversible reactions by narrowing the potential window. Devices of C9 and C10 were tested in the voltage range of −0.5 to +0.5 V considering that a symmetric potential window is normally accepted for the evaluation of a symmetric cell. The specific capacitance of device C9 shows better capacitance retention of about 72% after 5500 cycles. As to the device C10, the specific capacitance decreased quickly. Since no obvious irreversible reactions are observed, the capacitance degradation might be due to the decrease of ionic conductivity.25–27 Such high fluctuations in cycling stability of devices assembled indicate that the key issue causing the lower chemical and electrochemical stability of PBI–KOH requires further investigation. More detailed work is undergoing to reveal the degradation mechanism of PBI–KOH based electrolyte. Nevertheless, our results suggest that PBI–KOH electrolyte is still promising for ASSs.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is estimated to be close to the optimized value. Fig. 5a shows the cycling performance of the ASS tested within a potential window of 0–1.5 V at a current density of 0.4 A g−1. The highest specific capacitance obtained at fifth cycle reaches 118.6 F g−1 (Fig. 5a and b) based on the mass of active materials in two electrodes calculated by formula (2). It corresponds to a specific energy of 37.1 W h kg−1, calculated using the following eqn (3):
1, which is estimated to be close to the optimized value. Fig. 5a shows the cycling performance of the ASS tested within a potential window of 0–1.5 V at a current density of 0.4 A g−1. The highest specific capacitance obtained at fifth cycle reaches 118.6 F g−1 (Fig. 5a and b) based on the mass of active materials in two electrodes calculated by formula (2). It corresponds to a specific energy of 37.1 W h kg−1, calculated using the following eqn (3):
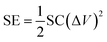 | (3) |
 | (4) |
Fig. 5a and b shows clear irreversible reactions during the charging process. The charging time is much longer than the discharging time. After 200 cycles, it becomes almost symmetric and keeps stable. It is worth to note that the trend of discharge specific capacitance of the assembled ASS is coincident with the combination of that of Ni(OH)2 (Fig. S5†) and AC (Fig. S3†). These results suggest that PBI–KOH is a promising candidate to fabricate of ASSs including symmetric and asymmetric (or hybrid) devices with high energy density. Besides, recent report suggests that both cations and anions take part in the charge–discharge process.33 Our system provides an ideal system to further investigate the charge–discharge mechanism since PBI–KOH is an anion (OH−) conductor.
4. Conclusions
In this paper, alkaline all slid supercapacitor devices are successfully assembled using PBI–KOH electrolyte. When PBI soaked with higher concentrated KOH solution, the assembled device gives better rate capability but suffers from lower cycling stability. Adding binder and narrowing the potential window are two useful ways to elevate the specific capacitance retention. The device C5 exhibits very promising cycling performance with the specific capacitance remaining roughly stable in the first 2000 cycles test. However, the key factor causing the loss of ionic conductivity and irreversible reactions is still not clear. Furthermore, an asymmetric (or hybrid) device based AC and Ni(OH)2 spheres has also been fabricated successfully and exhibits a relative high specific energy of 37.1 W h kg−1 at a current density of 0.4 A g−1. Although PBI–KOH suffers from the relatively low cycling stability currently, this work still demonstrates the feasibility of developing alkaline based ASSs using anion conducting polymer electrolytes for achieving high energy density.Acknowledgements
The authors acknowledge the financial support of the Fundamental Research Funds for the Central Universities (Grant No. 2015HGCH0001), the National Natural Science Foundation of China (Grant No. 21503065) and the second-class General Financial Grant from the China Postdoctoral Science Foundation (Grant No. 2015M571924). The Project is also sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry. The authors thank the staff in the Analytical and Testing Center of HFUT for their assistance in the materials characterization.Notes and references
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- L. L. Zhang and X. S. Zhao, Chem. Soc. Rev., 2009, 38, 2520–2531 RSC.
- M. Inagaki, H. Konno and O. Tanaike, J. Power Sources, 2010, 195, 7880–7903 CrossRef CAS.
- X. M. Li, Q. G. Li, Y. Wu, M. C. Rui and H. B. Zeng, ACS Appl. Mater. Interfaces, 2015, 7, 19316–19323 CAS.
- G. A. Snook, P. Kao and A. S. Best, J. Power Sources, 2011, 196, 1–12 CrossRef CAS.
- P. H. Yang and W. J. Mai, Nano Energy, 2014, 8, 274–290 CrossRef CAS.
- X. Cai, M. Peng, X. Yu, Y. P. Fu and D. C. Zou, J. Mater. Chem. C, 2014, 2, 1184–1200 RSC.
- X. L. Dong, Z. Y. Guo, Y. F. Song, M. Y. Hou, J. Q. Wang, Y. G. Wang and Y. Y. Xia, Adv. Funct. Mater., 2014, 24, 3405–3412 CrossRef CAS.
- D. P. Cole, A. L. M. Reddy, M. G. Hahm, R. McCotter, A. H. C. Hart, R. Vajtai, P. M. Ajayan, S. P. Karna and M. L. Bundy, Adv. Energy Mater., 2014, 4, 1300844 Search PubMed.
- G. Lee, D. Kim, D. Kim, S. Oh, J. Yun, J. Kim, S. S. Lee and J. S. Ha, Energy Environ. Sci., 2015, 8, 1764–1774 CAS.
- C. Z. Meng, C. H. Liu, L. Z. Chen, C. H. Hu and S. S. Fan, Nano Lett., 2010, 10, 4025–4031 CrossRef CAS PubMed.
- R. Z. Li, Y. M. Wang, C. Zhou, C. Wang, X. Ba, Y. Y. Li, X. T. Huang and J. P. Liu, Adv. Funct. Mater., 2015, 25, 5384–5394 CrossRef CAS.
- Z. Tang, C. H. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272–1278 CrossRef CAS.
- H. L. Wang, Y. Y. Liang, T. Mirfakhrai, Z. Chen, H. S. Casalongue and H. J. Dai, Nano Res., 2011, 4, 729–736 CrossRef CAS.
- D. Rathod, M. Vijay, N. Islam, R. Kannan, U. Kharul, S. Kurungot and V. Pillai, J. Appl. Electrochem., 2009, 39, 1097–1103 CrossRef CAS.
- R. S. Hastak, P. Sivaraman, D. D. Potphode, K. Shashidhara and A. B. Samui, Electrochim. Acta, 2012, 59, 296–303 CrossRef CAS.
- H. Gao and K. Lian, RSC Adv., 2014, 4, 33091–33113 RSC.
- J. Z. Song, J. H. Li, J. Y. Xu and H. B. Zeng, Nano Lett., 2014, 14, 6298–6305 CrossRef CAS PubMed.
- P. Sivaraman, R. K. Kushwaha, K. Shashidhara, V. R. Hande, A. P. Thakur, A. B. Samui and M. M. Khandpekar, Electrochim. Acta, 2010, 55, 2451–2456 CrossRef CAS.
- J. Yan, L. P. Yang, M. Q. Cui, X. Wang, K. J. J. Z. Chee, V. C. Nguyen, V. Kumar, A. Sumboja, M. Wang and P. S. Lee, Adv. Energy Mater., 2014, 4, 1400781 Search PubMed.
- A. L. M. Reddy, F. E. Amitha, I. Jafri and S. Ramaprabhu, Nanoscale Res. Lett., 2008, 3, 145–151 CrossRef.
- J. W. Cui, X. Y. Zhang, L. Tong, J. B. Luo, Y. Wang, Y. Zhang, K. Xie and Y. C. Wu, J. Mater. Chem. A, 2015, 3, 10425–10431 CAS.
- X. M. Li, L. F. Jiang, C. Zhou, J. P. Liu and H. B. Zeng, NPG Asia Mater., 2015, 7, e165 CrossRef CAS.
- S. Maurya, S. H. Shin, Y. Kim and S. H. Moon, RSC Adv., 2015, 5, 37206–37230 RSC.
- H. Y. Hou, G. Q. Sun, R. H. He, Z. M. Wu and B. Y. Sun, J. Power Sources, 2008, 182, 95–99 CrossRef CAS.
- Y. J. Wang, J. L. Qiao, R. Baker and J. J. Zhang, Chem. Soc. Rev., 2013, 42, 5768–5787 RSC.
- H. Y. Hou, S. L. Wang, Q. A. Jiang, W. Jin, L. H. Jiang and G. Q. Sun, J. Power Sources, 2011, 196, 3244–3248 CrossRef CAS.
- L. H. Dong, Y. Chu and W. D. Sun, Chem.–Eur. J., 2008, 14, 5064–5072 CrossRef CAS PubMed.
- G. W. He, Z. Li, J. Zhao, S. F. Wang, H. Wu, M. D. Guiver and Z. Y. Jiang, Adv. Mater., 2015, 27, 5280–5295 CrossRef CAS PubMed.
- J. Yan, E. Khoo, A. Sumboja and P. S. Lee, ACS Nano, 2010, 4, 4247–4255 CrossRef CAS PubMed.
- J. Yan, A. Sumboja, X. Wang, C. P. Fu, V. Kumar and P. S. Lee, Small, 2014, 10, 3568–3578 CrossRef CAS PubMed.
- C. X. Guo, A. A. Chitre and X. M. Lu, Phys. Chem. Chem. Phys., 2014, 16, 4672–4678 RSC.
- J. M. Griffin, A. C. Forse, W. Y. Tsai, P. L. Taberna, P. Simon and C. P. Grey, Nat. Mater., 2015, 14, 812–819 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26848f |
| This journal is © The Royal Society of Chemistry 2016 |






