Preparation and characterization of alginate/HACC/oyster shell powder biocomposite scaffolds for potential bone tissue engineering applications†
Tai-ying Chen,
Hao-chao Huang,
Jia-lin Cao,
Yan-jiao Xin,
Wen-feng Luo and
Ning-jian Ao*
Key Laboratory of Biomaterials of Guangdong Higher Education Institutes, Department of Biomedical Engineering, Jinan University, Guangzhou 510632, China. E-mail: taonj@jnu.edu.cn; Tel: +86 136 6059 6766
First published on 1st April 2016
Abstract
Tissue engineering scaffolds combining biominerals and natural polymers are prospective candidates for bone repair materials. In this work, biocompatible and antibacterial scaffolds were prepared by blending alginate, hydroxypropyl trimethyl ammonium chloride chitosan (HACC) and oyster shell powder (OSP) by freeze drying. SEM, FTIR, mechanical testing and BET surface area analysis were applied to characterize the prepared scaffolds. The swelling behavior, biomineralization, in vitro biodegradation, protein adsorption, antibacterial test, cytotoxicity, and alkaline phosphatase (ALP) activity test were also investigated. The prepared scaffolds have a highly porous and interconnected pore structure with a pore size of around 20 to 200 μm suitable for cells to grow in. The addition of oyster shell powder in the scaffolds improved their compressive strength, specific surface area, and protein adsorption capacity, and also controlled the swelling behavior. Both of the scaffolds were biodegradable; the degradation was decreased on increasing the OSP content in the scaffolds, while the biomineralization ability was improved. Meanwhile the prepared scaffolds exhibited antibacterial activity toward E. coli and S. aureus. There was no significant cytotoxicity effect of the prepared scaffolds towards MC3T3-E1, HOS, and MG-63 cells and they have a good ALP activity. Therefore, these results suggest that the Alg/HACC/OSP scaffold is a prospective candidate for bone repair materials.
1. Introduction
Autografts are the most commonly used treatment of bone defects and manifest great therapeutic effects in bone fusion.1 However, the donor supply limitation and site morbidity remain a significant challenge and limit its development. Although allograft and xenograft could overcome the drawback mentioned above, they may lead to pathogen transmission and immune rejection, respectively.2 Hence, synthetic materials with desirable biocompatibility and satisfactory mechanical properties, as well as other properties, are promising candidates for bone tissue engineering.3 Meanwhile, natural polysaccharides, such as chitin, chitosan, alginate, carrageenan and chondroitin sulfate etc., due to being biocompatible, biodegradable, non-toxic and abundant,4 have become widely used as versatile biomaterials for diverse biomedical applications.5,6 After chemical or physical modification, these polysaccharides can also be given suitable properties toward further bone tissue engineering applications.Alginate, as one of the natural anionic polysaccharides, consists of two monosaccharide units: guluronic acid and mannuronic acid. Due to good biocompatibility, degradability and non-cytotoxicity, it has been widely used for cell encapsulation, growth factor and drug delivery, and as a scaffold for tissue engineering.7–12 Alginate has been used for cartilage and bone tissue engineering,13 and there is considerable research demonstrating its advantages in long-term cultivation of osteocytes and chondrocytes.14,15 It has also been successfully applied in in vivo bone formation.16 However, the poor mechanical properties, lack of cellular interactions, and uncontrollable degradation of the alginate may limit its further application for tissue regeneration.17 Meanwhile, because of their hydrophilicity, alginate scaffolds have a poor protein adsorption capacity, which may result in a low cell attachment. Thus, to overcome these defects, multicomponent biocomposite scaffolds need to be constructed.
Oyster shell (OS), as a waste product from mariculture, is mainly composed of calcium carbonate CaCO3 and other minerals in trace quantities. Due to the wide availability and low cost of oyster shell, it has been widely use in construction materials, biological aerated filters and adsorbents for phosphate.18–20 There is some research showing that the formation process of the oyster shell is similar to osteogenesis in the human body.21,22 Many studies23–27 have indicated that sea shell exhibits desirable biocompatibility, osteogenic ability, and slow re-absorption rates in vivo. Shen et al.27 reported that nacre contains several signaling molecules, like bone morphogenetic proteins (BMPs), which is one of the bone growth factors existing in human bone, that can activate the osteogenetic bone marrow cells both in vivo and in vitro and lead to bone formation. Mouriès et al.28 reported that a nacre organic matrix (WSM) of a bivalve mollusk contains signal molecules that can stimulate the osteogenic pathway in mammalian cells that are targets for bone induction. Therefore, oyster shell could be a prospective substitute for bone repair materials, and it is sensible to incorporate oyster shell powder into alginate to produce a composite with improved properties, and the composite can be a prospective candidate for bone repair materials.
Postoperative inflammation causes bacterial colonization of implanted materials which may lead to implantation failure. Some researchers have loaded an antibacterial substance such as antibiotics and silver into the scaffolds which can minimize the risk of orthopedic infection. Chitosan, obtained by de-acetylation of chitin, has desirable biocompatibility and biodegradability, and is non-toxic, and there is considerable research reporting that chitosan holds a broad spectrum of antimicrobial properties.29–32 However, the poor hydrophilicity of chitosan limits its further applications as an antimicrobial agent. To overcome this drawback of chitosan, quaternary ammonium chitosan that contains a series of quaternary ammonium substitutions has been synthesized, and its antibacterial activities have been demonstrated to significantly improve with the increase of the alkyl substituent chain length.33–37 Among these, hydroxypropyl trimethyl ammonium chloride chitosan (HACC) exhibited a desirable antibacterial activity with long duration, which makes it an expected candidate for using in bone repair.38
To date, there is no previous research reporting the incorporation of both HACC and oyster shell powder into alginate scaffolds, and investigating their physical and chemical properties, biomineralization capability and antibacterial ability. Thus, in our work, biocomposite scaffolds containing alginate, hydroxypropyl trimethyl ammonium chloride chitosan and oyster shell powder (Alg/HACC/OSP) were prepared by the freeze drying method. The scaffolds were characterized in terms of their morphology and microstructure, physicochemical properties, swelling behavior, in vitro biodegradation and mineralization, protein adsorption, antibacterial ability, and biocompatibility.
2. Materials and methods
2.1 Materials
Alginate sodium was purchased from Dongyue Biotech-Ltd., China. Oyster shell was obtained from Zhuhai Shell Technology Co., Ltd., China. N-2-Hydroxypropyl trimethyl ammonium chloride chitosan (HACC), Dulbecco's Modified Eagle Medium (DMEM) and albumin were supplied by Jianyang Biotech-Ltd., China. Sodium hydroxide and other reagents used were all of analytical grade purchased from Sigma-Aldrich, USA.2.2 Preparation of oyster shell powder
The preparation of oyster shell powder according to the method of Li et al.39 is as follows. Briefly, oyster shell was washed to remove the attachments. In order to obtain the nacre, the oyster shell was soaked into 5% sodium hydroxide solution for 48 h, and ultrasonicated to separate the prismatic layer then filtered to obtain the nacre. Then the nacreous layer was dried at 100 °C overnight and ground using a stirring ball mill (DECO-PBM-H-0.4L), using zirconia balls as milling media with a ball-to-powder mass ratio of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and at a speed of 100 rpm for 24 h, obtaining the raw oyster shell powder. The raw oyster shell powder was slurried with distilled water in a 0.5 kg L−1 ratio and the slurry was further ground using a high shear powder liquid mixing emulsifying machine (SME 65) at a rotational speed of 2500 rpm for 8 h. The resultant slurry was filtered, dried, and scattered again using the stirring ball mill to obtain the oyster shell powder.
1 and at a speed of 100 rpm for 24 h, obtaining the raw oyster shell powder. The raw oyster shell powder was slurried with distilled water in a 0.5 kg L−1 ratio and the slurry was further ground using a high shear powder liquid mixing emulsifying machine (SME 65) at a rotational speed of 2500 rpm for 8 h. The resultant slurry was filtered, dried, and scattered again using the stirring ball mill to obtain the oyster shell powder.
2.3 Preparation of the Alg/HACC/OSP and Alg/HACC scaffolds
The scaffolds containing HACC, alginate, and oyster shell powder (OSP) were prepared by the freeze drying method. Briefly a HACC (5% w/v) solution was prepared by dissolving the HACC in deionized water. 5% alginate solutions were prepared for the dispersion of OSP by dissolving the alginate in deionized water. The dispersion of OSP was facilitated by a magnetic stirrer for 2 h and subsequent sonication for 2 h. Alginate/HACC/OSP composite scaffolds were prepared by mixing the alginate/OSP mixture with 5% HACC solution and stirring for an additional hour at 1000 rpm. The OSP content of the alginate/HACC/OSP composite scaffolds ranged from 1.2% to 4.8% (wt), and named as Alg/HACC/OSP1.2, Alg/HACC/OSP2.4, Alg/HACC/OSP3.6, and Alg/HACC/OSP4.8. The resultant mixture was then placed into a module and frozen overnight at −80 °C, and the dried scaffolds were obtained by freeze drying in a lyophilizer. The resultant scaffolds were soaked in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of CaCl2 solution 10% (w/v) and ethanol for 1 hour and then put into a lyophilizer again. Simultaneously, to fabricate the Alg/HACC scaffolds, the above procedures were followed in which the addition of OSP was excluded, and this scaffold was also named as Alg/HACC/OSP0. The obtained scaffolds were kept in a dryer for further analysis.
1 ratio of CaCl2 solution 10% (w/v) and ethanol for 1 hour and then put into a lyophilizer again. Simultaneously, to fabricate the Alg/HACC scaffolds, the above procedures were followed in which the addition of OSP was excluded, and this scaffold was also named as Alg/HACC/OSP0. The obtained scaffolds were kept in a dryer for further analysis.
2.4 Characterization
The morphology of the Alg/HACC and Alg/HACC/OSP scaffolds was investigated by scanning electron microscopy (Philips XL-30, Netherlands). The FT-IR spectra were recorded on a FT-IR spectrophotometer (American Perkin Elmer Co., USA) using a KBr press. The scan range was from 400–4000 cm−1. The compressive strength and Young's modulus of the prepared scaffolds were measured on a mechanical testing machine (Instron 3369, USA) with a loading rate of 0.5 mm min−1, and the specimens for the test were cylinders with a diameter of 10 mm and a height of 10 mm. The Brunauer–Emmett–Teller (BET) surface area and BJH pore size distribution of the prepared scaffolds were determined by N2 adsorption–desorption isotherms using a V-sorb 2800TP surface analyzer at 77 K. All the samples were degassed (at 393 K for 3 h) before the measurements.2.5 In vitro swelling studies
The swelling behavior of the prepared scaffolds was measured by soaking the samples into 1× PBS (phosphate buffered saline) at 37 °C and pH 7.4 for 24 h, 48 h and 72 h. After each incubation period, the scaffolds were removed from the solution and weighed after removing the surface water of the samples by filter paper. The swelling ratio was calculated according to:| Swelling ratio = (Ww − Wd)/Wd × 100% | (1) |
2.6 In vitro mineralization and biodegradation
The in vitro mineralization of the prepared scaffolds was measured by the simulated body fluid (SBF) method. The SBF solution was prepared according to a previous report.40 The prepared scaffolds (1 × 1 × 1 cm3) were soaked in 50 mL simulated body fluid (SBF) and then incubated at 37 °C for 1 day, 7 days and 14 days. The formation of the apatite was characterized by SEM (Philips XL-30, Netherlands) and XRD (Bruker D8 Advance, Germany).The degradation rate of the prepared scaffolds was evaluated by the following process: the prepared scaffolds (1 cm × 1 cm × 1 cm) were immersed in SBF solution at 37 °C and incubated for 24 h, 48 h and 72 h. After each incubation period, the scaffolds were removed from the solution and dried at 70 °C until they were of a constant weight. The degradation percentage was calculated according to:
| Degradation% = (Wi − Wt)/Wi × 100% | (2) |
2.7 Protein adsorption
Albumin was used as a standard protein to investigate the protein adsorption to the prepared scaffolds. All the specimens were sterilized by UV exposure for 30 min. The specimens were then soaked in albumin solution (5 mg mL−1) at 37 °C and incubated for 24 h and 48 h. After each incubation period, the specimens were separated from the solution and rinsed with deionized water 3 times to remove the non-adherent proteins. In order to elute the adsorbed protein the specimens were placed into a 24-well plate and incubated with 1% sodium dodecyl sulfate (SDS) solution for 1 h. After the incubation, the SDS solution was collected and then measured by BCA assay using a plate reader (Multiskan MK3, Thermo) at a wavelength of 570 nm. The test was carried out in triplicate, and five specimens were used in each group in each experiment.2.8 Antibacterial activity test
The antibacterial activity of the prepared scaffolds against E. coli and S. aureus was determined by the agar dilution method and shake-flask method.For the agar dilution method, E. coli (1 × 108 CFU mL−1) and S. aureus (1 × 108 CFU mL−1) were seeded over agar plates, respectively, then the prepared scaffolds (disks 5 mm in diameter and 2 mm in thickness) were placed on top. The incubation was done at 37 °C for 24 h.
For the shake-flask method, 100 mg of each sample was cut into small pieces, sterilized by UV light, and then dispersed in 9 mL of sterile saline water (0.85 wt%). 1 mL of bacterial (E. coli or S. aureus) culture (108 CFU mL−1) was subsequently added to this solution and finally the concentration of the sample in the suspension reached 10 mg mL−1. The flask was shaken at 90 rpm for 24 h, and the temperature was balanced at 37 °C. Blanks without the prepared scaffolds were also run. The surviving bacteria before and after shaking were counted by the plate count method. The relative colony forming units (CFU) were calculated as follows:
| Relative CFU% = CFU(24 h)/CFU(0 h) × 100% | (3) |
2.9 Cell culture
MC3T3-E1, human osteosarcoma cells (HOS) and MG-63 cells, provided by Medical Laboratory, Jinan University, China, were selected for evaluation. MC3T3-E1, HOS, and MG-63 cells were seeded in Dulbecco's Modified Eagle Medium (DMEM, Gibco, U.S.A.) supplemented with 10% (v/v) fetal bovine serum (FBS, Gibco, U.S.A.) at 37 °C in a humidified atmosphere with 5% CO2, then detached with 0.25% trypsin/0.03% ethylene diamine tetraacetic acid (EDTA). The cell density was calculated, allowing for the desired density to be used in later experiments.2.10 Cytotoxicity studies
The cytotoxicity study of the prepared scaffolds was investigated using a Cell Counting Kit-8 (CCK8) assay. Samples (5 × 5 mm2) were sterilized by ultraviolet (UV) light, and then placed in culture medium overnight. MC3T3-E1 cells were seeded onto scaffolds at a density of 1 × 105 per sample, and incubated at 37 °C in an atmosphere of 5% CO2. The medium was changed every second day. After incubation for 24 and 48 h, 50 μL of CCK-8 solution was added to each well, and incubated for another 4 hours. The absorbance was measured at 570 nm using a plate reader (Multiskan MK3, Thermo). The experiment was repeated 5 times. The cytotoxicity study to the HOS and MG-63 followed the procedure mentioned above. The control group is a pure cell culture, exclusively involving cells and a medium. The cell relative growth rate was determined using the formula:| RGR% = (ODsample − ODNegative control)/ODNegative control × 100% | (4) |
2.11 Alkaline phosphatase (ALP) activity test
The prepared scaffolds (5 × 5 mm2) were sterilized by ultraviolet (UV) light and then placed in a 24-well plate. 1 mL of medium and a MG-63 cell suspension at a density of 1 × 105 cells per well were then added to each well, after which the contents are cultured in an incubator for 7 days and 14 days. Cells were rinsed twice with phosphate buffered saline (PBS) and then fixed with 4% paraformaldehyde. An ALP substrate mixture was then added and incubated for 10 min. The ALP activity was quantified at a wavelength of 405 nm using a microplate reader (SPECTRA max 384, Molecular Devices) as the substrate, and the total protein content in the cell lysate was determined by BCA assay using a plate reader (Multiskan MK3, Thermo) at a wavelength of 570 nm and calculated according to a series of albumin (bovine serum albumin) standards. The ALP levels were normalized to the total protein content and performed in quadruplicate for all of the experiments.2.12 Statistical analysis
All the results are expressed as the mean ± standard deviation (SD). Experiments were repeated three times unless otherwise specified. One-way ANOVA was performed to analyze the variables, and p < 0.05 was considered statistically significant.3. Results and discussion
Scaffolds for bone engineering should have a well defined interconnected pore structure that can provide a biological environment for cell adhesion and proliferation as well as blood vessels and bone growth.41 The microstructure of the prepared scaffolds was investigated using scanning electron microscopy (Fig. 1). As observed, both composite scaffolds had an interconnected highly porous architecture. The pore size of the composite scaffolds was around 20 to 200 μm, which is favorable for cell adhesion and proliferation as well as new bone tissue growth.42,43 Fig. 1(A1–E1) show the porous structures of the prepared scaffolds and reveal the porous interconnection. In addition, Fig. 1(B2–E2) show the good distribution of OSP in the Alg/HACC/OSP scaffolds, and it can be seen that the OSP is exposed on the wall of the composite scaffolds which enables the OSP to contact the body fluid directly. Therefore, it will improve the mechanical strength and biocompatibility of the prepared scaffold. | ||
| Fig. 1 SEM image of Alg/HACC scaffolds (A1, A2), Alg/HACC/OSP1.2 scaffolds (B1, B2), Alg/HACC/OSP2.4 scaffolds (C1, C2), Alg/HACC/OSP3.6 scaffolds (D1, D2), and Alg/HACC/OSP4.8 scaffolds (E1, E2). | ||
Alginate is an anionic polymer which could have strong electrostatic interaction with hydroxypropyl trimethyl ammonium chloride chitosan. These electrostatic interactions were investigated using FT-IR analysis. In Fig. 2, there are the FT-IR spectra of the prepared scaffolds and the components used in the scaffold fabrication. The FT-IR spectrum of alginate shows a characteristic peak at 1625 cm−1, corresponding to the carboxyl group (COO−); while the spectrum of HACC shows characteristic peaks at 1480 cm−1 and 1375 cm−1 corresponding to the bending vibration of –CH3 and the stretching vibration of C–N+ for the quaternary ammonium salt group, respectively. The OSP spectrum shows its characteristic peak at 1792 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of the carbonate ions, and the peaks at 1435 cm−1 (V3), 1038 cm−1 (V1), 876 cm−1 (V2) and 710 cm−1 (V4) correspond to the internal vibration modes of the carbonate ions. The splitting of V4 is a characteristic aragonite structure,44 and the strongest absorption band of the OSP in the V3 region was overlapped by the absorption of the organic matrix. Compared to the spectra of HACC and alginate, there are some significant changes found in curve (H). The peak at 1625 cm−1 (COO−) present for alginate and the peaks at 1482 cm−1 (C–H) and 1300 cm−1 (C–N+) for HACC are shifted to 1605 cm−1, 1412 cm−1, and 1301 cm−1 respectively, demonstrating the strong groups of HACC and carboxyl groups of alginate.
O groups of the carbonate ions, and the peaks at 1435 cm−1 (V3), 1038 cm−1 (V1), 876 cm−1 (V2) and 710 cm−1 (V4) correspond to the internal vibration modes of the carbonate ions. The splitting of V4 is a characteristic aragonite structure,44 and the strongest absorption band of the OSP in the V3 region was overlapped by the absorption of the organic matrix. Compared to the spectra of HACC and alginate, there are some significant changes found in curve (H). The peak at 1625 cm−1 (COO−) present for alginate and the peaks at 1482 cm−1 (C–H) and 1300 cm−1 (C–N+) for HACC are shifted to 1605 cm−1, 1412 cm−1, and 1301 cm−1 respectively, demonstrating the strong groups of HACC and carboxyl groups of alginate.
Scaffolds for bone regeneration must provide sufficient mechanical strength for in vivo applications. Fig. 3 shows the compressive strengths and the Young's modulus of the Alg/HACC, Alg/HACC/OSP1.2, Alg/HACC/OSP2.4, Alg/HACC/OSP3.6 and Alg/HACC/OSP4.8 samples. The compressive strengths of the samples range from 3.04 to 8.08 MPa and the Young's modulus of the samples ranges from 13.21 to 60.37 MPa. The results indicate that the compressive strengths and Young's modulus of the samples increased with the increasing OSP content in the scaffolds. It is known that the compressive strength of cancellous bone is about 2–12 MPa. The compressive strength and Young's modulus of the prepared scaffolds are comparable with those of cancellous bone, indicating that the mechanical strength of the Alg/HACC/OSP scaffold is sufficiently high for some non-load-bearing applications of bone repair.45
The specific surface areas of the prepared scaffolds were measured at 77 K by N2 adsorption–desorption isotherms according to the BET method. The N2 adsorption–desorption isotherms and pore size distributions of the prepared scaffolds are shown in Fig. 4. The pore size distribution curve was calculated using the Barrett–Joyner–Halenda (BJH) equation from the desorption branch of the isotherm. Fig. 4 shows that the N2 adsorption–desorption isotherms of the prepared scaffolds exhibit a typical IV isotherm, indicating that both of the prepared scaffolds possess a mesoporous structure. The corresponding specific surface area, average pore size, pore volume and total pore volume of the prepared scaffolds are listed in Table 1. It could be observed that the Alg/HACC/OSP4.8 scaffold exhibits the highest BET surface area among all the samples, and the BET surface area of the prepared scaffolds increased with increasing the content of OSP in the scaffolds. The obtained BET surface area and pore size distribution indicate that the introduction of OSP in the scaffolds could improve the surface area of the scaffolds.
| Sample | Surface area (m2 g−1) | Pore size (nm) | Pore volume (cm3 g−1) | Total pore volume (cm3 g−1) |
|---|---|---|---|---|
| Alg/HACC | 69.2 | 10.1 | 0.0043 | 0.023 |
| Alg/HACC/OSP1.2 | 71.4 | 9.5 | 0.0039 | 0.028 |
| Alg/HACC/OSP2.4 | 73.9 | 9.3 | 0.0038 | 0.026 |
| Alg/HACC/OSP3.6 | 87.7 | 8.2 | 0.0036 | 0.031 |
| Alg/HACC/OSP4.8 | 97.1 | 7.4 | 0.0027 | 0.037 |
For bone tissue engineering scaffolds, the swelling behavior must be investigated because it will be surrounded by biological fluids in in vivo applications. Most natural polymers, including alginate, swell readily in biological fluids. It was reported that alginate has a high swelling capacity, and it can absorb water quickly and holds 200–300 times its own weight.46 In vitro culture studies suggested that initial swelling leads to the pore size increasing which is beneficial to the cell adhesion and proliferation in the scaffolds.47 However, the decline of mechanical strength and compressive stress to the surrounding tissue may cause the constant swelling. The swelling behaviors of the Alg/HACC and Alg/HACC/OSP scaffolds were investigated by soaking the prepared scaffolds in PBS solution at 37 °C for 24, 48 and 72 h. As shown in Fig. 5(A), the Alg/HACC scaffolds experienced the highest swelling ratio for all incubation times, and the swelling ratio decreased with the increasing content of OSP in the scaffolds. And the swelling ratio between these specimens slightly increases with the increase of immersing time (Fig. 5(A)). The addition of oyster shell powder would obstruct the permeation of PBS solution, thus a decrease of the swelling ratio was prominently observed. Although the Alg/HACC/OSP scaffolds exhibit a lower swelling ratio compared to that of the Alg/HACC scaffolds, the presence of oyster shell powder in the scaffolds could increase the surface area which is beneficial to the cell attachment and protein adsorption.
Implanted materials absorb different proteins from body fluids and the surrounding tissue, which plays an important role in cell proliferation and differentiation.48,49 The protein adsorption of the prepared scaffolds after 24 h and 48 h incubation with albumin solution was evaluated using a BCA assay and the results are shown in Fig. 5(B). Compared with the Alg/HACC scaffold, the Alg/HACC/OSP scaffolds show a higher protein adsorption after 24 h incubation. After 48 h incubation, it was clearly observed that the quantity of protein being adsorbed in the scaffolds was significantly increased, and the quantity of protein being adsorbed in the Alg/HACC/OSP scaffolds was larger than the protein adsorption of the Alg/HACC scaffold. And the results also revealed that the protein adsorption of the Alg/HACC/OSP scaffolds increased with the increasing amount of oyster shell powder in the Alg/HACC/OSP scaffolds. The BET test indicated that the additive OSP increases the surface area of the Alg/HACC/OSP scaffolds, which greatly improved the protein adsorption ability of the Alg/HACC/OSP scaffolds. In addition, the OSP has a higher surface to volume ratio, which can provide more sites for protein binding which may increase cell adhesion. Thus, the addition of oyster shell powder would improve the osteoblasts adhesion and the osteogenic ability.
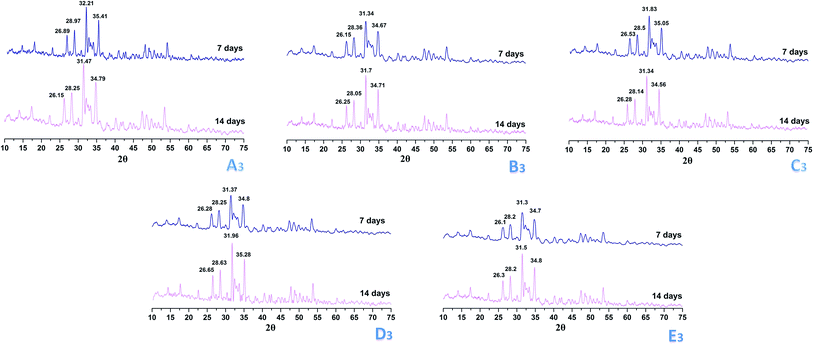
Biomineralizaton ability is an essential factor to promote the bone-binding ability and osteogenic ability for bone repair materials. The biomineralization of the prepared scaffolds was investigated by soaking the scaffolds in SBF solution. The surface morphologies of the Alg/HACC scaffolds and Alg/HACC/OSP scaffolds after 7 days and 14 days incubation with SBF solution were observed by SEM (Fig. 6). There is no significant different in the deposition of the apatite layer on the surfaces of the Alg/HACC/OSP scaffolds and Alg/HACC scaffold after 7 days incubation. After 14 days incubation, the Alg/HACC/OSP scaffolds showed an obvious and increased amassment of apatite compared to that of the Alg/HACC scaffolds. The presence of oyster shell powder in the Alg/HACC/OSP scaffolds initiated the formation of a calcium phosphate layer due to the release of calcium ions from the oyster shell powder surface, and thus the oyster shell powder surface is activated in situ where the calcium ions are released. The free calcium ions bind the phosphate ions in the SBF solution and precipitate it again at the active sites on the Alg/HACC/OSP scaffolds in the form of hydroxyapatite. In order to identify the deposits on the scaffolds, XRD measurements were also conducted. The XRD patterns (Fig. 7) of the prepared scaffolds collected after 7 days and 14 days incubation exhibit several particular peaks corresponding to the characteristic peak of hydroxyapatite deposition. The observed diffraction peaks are identified by the standard JCPDS (file no. 09-0432) file which indicates the highly crystalline form of hydroxyapatite, indicating the presence of hydroxyapatite growth on the specimens' surface.
 | ||
| Fig. 6 SEM images on the surface of the prepared scaffolds after soaking in SBF for 7 days and 14 days. | ||
Biodegradability and controlled degradation rate are essential for bone repair materials. The degradation behavior of the prepared scaffolds was investigated in SBF solution, as shown in Fig. 8. The degradation rate of all the specimens was linear with the increasing incubation time. And it can be clearly observed that the Alg/HACC scaffolds have the highest degradation rate after different incubation times. However, the degradation of the Alg/HACC/OSP scaffolds was significantly delayed, and the degradation rate of the Alg/HACC/OSP scaffolds was inversely proportional to the OSP content in the scaffolds. After 14 days immersion, the Alg/HACC/OSP4.8 scaffold exhibited the lowest degradation rate among all the specimens. The delayed degradation rate of the Alg/HACC/OSP scaffolds may be attributed to the low solubility of the OSP in the scaffolds, and the embedded OSP particles on the scaffold surface obstruct the permeation of SBF solution. In addition, the degradation of the Alg/HACC scaffold and Alg/HACC/OSP scaffolds consisted of two processes: the degradation of the scaffolds and the formation of hydroxyapatite on the scaffold surface. The result of the in vitro mineralization test indicated that the addition of OSP improved the formation of the hydroxyapatite on the scaffold surface. This may lead to a reduction of the degradation rate of the Alg/HACC/OSP scaffolds.
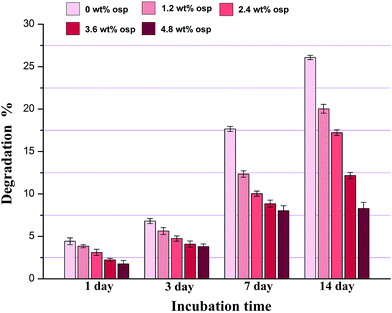 | ||
| Fig. 8 In vitro biodegradation of Alg/HACC and Alg/HACC/OSP scaffolds in SBF solution at 37 °C for 1 day, 3 days, 7 days and 14 days. | ||
It is well known in the surgery field that orthopedic interventions have a high risk of triggering inflammatory reactions caused by bacterial colonization of the biomaterial surface to be implanted. Such conditions can be responsible for implant failure, prolonged hospitalization periods, increased costs, and, in extreme cases, patient death.50 Therefore, in this work, the antibacterial activity of the scaffolds against S. aureus and E. coli were determined by the agar dilution method and shake-flask method. The results show that the scaffolds promote the inhibition of S. aureus and E. coli growth, where an inhibitory halo around the scaffold can be observed clearly (Fig. 9). And the relative CFU towards both E. coli and S. aureus illustrates that the prepared scaffolds can efficiently inhibit the proliferation of bacteria (Fig. 10). However, the addition of OSP did not alter the antibacterial activity of the scaffolds, as demonstrated by the inhibitory zone and the relative CFU which have no significant increases. The HACC, used in the production of the scaffolds, can interact with the electronegative residues present at the bacteria surface, increasing the cell wall permeability and therefore the leakage of intracellular constituents, thus promoting the dissipation of ionic gradients with bacteria.51 Furthermore, HACC can also create a barrier on the surface of the bacteria, preventing nutrients from entering the cell.52
Biomaterials intended for in vivo application should exhibit non-toxicity to mammalian cells. Thus, the Alg/HACC and Alg/HACC/OSP scaffolds were subjected to cytotoxicity using a Cell Counting Kit-8 (CCK-8) assay on osteoblast cells (MC3T3-E1), human osteoblastic cells (MG-63) and human osteosarcoma cell lines (HOS). The results of the CCK-8 assay (Fig. 11) reveal that the prepared scaffolds were not toxic to these three types of cells after incubation for 24 h and 48 h. Furthermore, it can be also noticed that the Alg/HACC/OSP1.2 scaffold has the highest cell viability among the samples towards these three types of cells.
 | ||
| Fig. 11 Cytotoxicity assay of the prepared scaffolds using (A) MC3T3-E1, (B) HOS and (C) MG-63, and the ALP activity of the prepared scaffolds (D). | ||
To better understand the effect of the prepared scaffolds on the behavior of the osteoblastic cells, osteoblast differentiation is one of the most important steps for overall cellular activity and thus bone formation ability. Thus, the effects of the prepared scaffolds on osteoblast differentiation were investigated using an alkaline phosphate activity (ALP) assay, which is an early maker of osteoblast differentiation. Fig. 11D shows the ALP activity of MG-63 cells cultured on the prepared scaffolds for 7 days and 14 days. The cells cultured on the Alg/HACC/OSP scaffolds for 7 days and 14 days exhibited a significantly higher ALP activity than that of the Alg/HACC scaffolds. And the ALP activity of the cells on the prepared scaffolds significantly increased with the increasing content of OSP in the scaffolds. In addition, the ALP activity value of the cells cultured on the Alg/HACC/OSP4.8 scaffolds was approximately 2.9 times higher than that on the Alg/HACC scaffolds for 7 days, and 3.3 times that for 14 days. It suggests that the introduction of OSP could up-regulate the ALP activity.
The results of the cytotoxicity test and the ALP activity test indicated that the prepared scaffolds have good biocompatibility and osteoblast differentiation ability, which can make them a promising candidate for bone regeneration.
4. Conclusions
In this research, biocomposite scaffolds containing alginate, hydroxypropyl trimethyl ammonium chloride chitosan and oyster shell powder were fabricated by lyophilization. SEM, FT-IR, mechanical testing and BET specific surface area analysis were applied to characterize the prepared scaffolds. The swelling behavior, biomineralization, in vitro biodegradation, protein adsorption, antibacterial test, cytotoxicity and alkaline phosphatase activity were also investigated. The results show that the prepared scaffolds have a highly porous and interconnected structure which is favorable for cell attachment and the growth of new tissue. And the oyster shell powder has a good distribution in the prepared scaffolds, thus improving the mechanical properties and the surface area of the Alg/HACC/OSP scaffolds. With the increase in OSP content in the scaffold, both the swelling ratio and degradation rate were found to decrease, while the biomineralization capability and protein adsorption capability were improved. In addition, the result also revealed that the Alg/HACC/OSP scaffolds exhibited good biocompatibility and effective antibacterial activity. Overall, the resultant scaffolds might be promising candidates for bone regeneration.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 209760868/B060805) and the Science and Technology Program of Guangdong Province.References
- K. J. Burg, S. Porter and J. F. Kellam, Biomaterial developments for bone tissue engineering, Biomaterials, 2000, 21, 2347–2359 CrossRef CAS PubMed.
- E. M. Younger and M. W. Chapman, Morbidity at bone graft donor sites, J. Orthop. Trauma, 1989, 3, 192–195 CrossRef CAS PubMed.
- K. S. Katti, Biomaterials in total joint replacement, Colloids Surf., B, 2004, 39, 133–142 CrossRef CAS PubMed.
- T. Coviello, P. Matricardi, C. Marianecci and F. Alhaique, Polysaccharide hydrogels for modified release formulations, J. Controlled Release, 2007, 119, 5–24 CrossRef CAS PubMed.
- F. Khan and S. R. Ahmad, Polysaccharides and their derivatives for versatile tissue engineering application, Macromol. Biosci., 2013, 13, 395–421 CrossRef CAS PubMed.
- K. Y. Lee, L. Jeong, Y. O. Kang, S. J. Lee and W. H. Park, Electrospinning of polysaccharides for regenerative medicine, Adv. Drug Delivery Rev., 2009, 61, 1020–1032 CrossRef CAS PubMed.
- C. Du, J. Zhao, J. Fei, L. Gao, W. Cui, Y. Yang and J. Li, Alginate-based microcapsules with a molecule recognition linker and photosensitizer for the combined cancer treatment, Chem.–Asian J., 2013, 8, 736–742 CrossRef CAS PubMed.
- W. Cui, Y. Cui, J. Zhao and J. Li, Fabrication of tumor necrosis factor-related apoptosis inducing ligand (TRAIL)/ALG modified CaCO3 as drug carriers with the function of tumor selective recognition, J. Mater. Chem. B, 2013, 1, 1326–1332 RSC.
- H. H. Tønnesen and J. Karlsen, Alginate in drug delivery systems, Drug Dev. Ind. Pharm., 2002, 28, 621–630 CrossRef PubMed.
- M. George and T. E. Abraham, Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review, J. Controlled Release, 2006, 114, 1–14 CrossRef CAS PubMed.
- I. Freeman and S. Cohen, The influence of the sequential delivery of angiogenic factors from affinity-binding alginate scaffolds on vascularization, Biomaterials, 2009, 30, 2122–2131 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Alginate: properties and biomedical applications, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS PubMed.
- K. T. Paige, L. G. Cima, M. J. Yaremchuk, J. P. Vacanti and C. A. Vacanti, Injectable cartilage, Plast. Reconstr. Surg., 1995, 96, 1390–1398 CAS.
- M. E. Oest, K. M. Dupont, H. J. Kong, D. J. Mooney and R. E. Guldberg, Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects, J. Orthop. Res., 2007, 25, 941–950 CrossRef CAS PubMed.
- X. Cai, Y. Lin, G. Ou, E. Luo, Y. Man, Q. Yuan and P. Gong, Ectopic osteogenesis and chondrogenesis of bone marrow stromal stem cells in alginate system, Cell Biol. Int., 2007, 31, 776–783 CrossRef CAS PubMed.
- A. D. Augst, H. J. Kong and D. J. Mooney, Alginate hydrogels as biomaterials, Macromol. Biosci., 2006, 6, 623–633 CrossRef CAS PubMed.
- K. Bouhadir and D. Mooney, Synthesis of hydrogels: alginate hydrogels, Methods of tissue engineering, 2002, pp. 653–662 Search PubMed.
- G.-L. Yoon, B.-T. Kim, B.-O. Kim and S.-H. Han, Chemical–mechanical characteristics of crushed oyster-shell, Waste Manag., 2003, 23, 825–834 CrossRef CAS PubMed.
- H.-B. Kwon, C.-W. Lee, B.-S. Jun, S.-Y. Weon and B. Koopman, Recycling waste oyster shells for eutrophication control, Resour., Conserv. Recycl., 2004, 41, 75–82 CrossRef.
- Y.-X. Liu, T. O. Yang, D.-X. Yuan and X.-Y. Wu, Study of municipal wastewater treatment with oyster shell as biological aerated filter medium, Desalination, 2010, 254, 149–153 CrossRef CAS.
- J. Balmain, B. Hannoyer and E. Lopez, Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction analyses of mineral and organic matrix during heating of mother of pearl (nacre) from the shell of the mollusc Pinctada maxima, J. Biomed. Mater. Res., 1999, 48, 749–754 CrossRef CAS PubMed.
- A. S. Mount, A. Wheeler, R. P. Paradkar and D. Snider, Hemocyte-mediated shell mineralization in the eastern oyster, Science, 2004, 304, 297–300 CrossRef CAS PubMed.
- M. Lamghari, M. Almeida, S. Berland, H. Huet, A. Laurent, C. Milet and E. Lopez, Stimulation of bone marrow cells and bone formation by nacre: in vivo and in vitro studies, Bone, 1999, 25, 91S–94S CrossRef CAS PubMed.
- M. Lamghari, S. Berland, A. Laurent, H. Huet and E. Lopez, Bone reactions to nacre injected percutaneously into the vertebrae of sheep, Biomaterials, 2001, 22, 555–562 CrossRef CAS PubMed.
- H. Liao, H. Mutvei, L. Hammarström, T. Wurtz and J. Li, Tissue responses to nacreous implants in rat femur: an in situ hybridization and histochemical study, Biomaterials, 2002, 23, 2693–2701 CrossRef CAS PubMed.
- M. Rousseau, L. Pereira-Mouriès, M.-J. Almeida, C. Milet and E. Lopez, The water-soluble matrix fraction from the nacre of Pinctada maxima produces earlier mineralization of MC3T3-E1 mouse pre-osteoblasts, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2003, 135, 1–7 Search PubMed.
- Y. Shen, J. Zhu, H. Zhang and F. Zhao, In vitro osteogenetic activity of pearl, Biomaterials, 2006, 27, 281–287 CrossRef CAS PubMed.
- L. P. Mouriès, M.-J. Almeida, C. Milet, S. Berland and E. Lopez, Bioactivity of nacre water-soluble organic matrix from the bivalve mollusk Pinctada maxima in three mammalian cell types: fibroblasts, bone marrow stromal cells and osteoblasts, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2002, 132, 217–229 CrossRef.
- L. R. Martinez, M. R. Mihu, M. Tar, R. J. Cordero, G. Han, A. J. Friedman, J. M. Friedman and J. D. Nosanchuk, Demonstration of antibiofilm and antifungal efficacy of chitosan against candidal biofilms, using an in vivo central venous catheter model, J. Infect. Dis., 2010, 201, 1436–1440 CrossRef CAS PubMed.
- E. Costa, S. Silva, C. Pina, F. Tavaria and M. Pintado, Evaluation and insights into chitosan antimicrobial activity against anaerobic oral pathogens, Anaerobe, 2012, 18, 305–309 CrossRef CAS PubMed.
- S.-Y. Ong, J. Wu, S. M. Moochhala, M.-H. Tan and J. Lu, Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties, Biomaterials, 2008, 29, 4323–4332 CrossRef CAS PubMed.
- M. Kong, X. G. Chen, K. Xing and H. J. Park, Antimicrobial properties of chitosan and mode of action: a state of the art review, Int. J. Food Microbiol., 2010, 144, 51–63 CrossRef CAS PubMed.
- C. H. Kim, J. W. Choi, H. J. Chun and K. S. Choi, Synthesis of chitosan derivatives with quaternary ammonium salt and their antibacterial activity, Polym. Bull., 1997, 38, 387–393 CrossRef CAS.
- Z.-X. Peng, L. Wang, L. Du, S.-R. Guo, X.-Q. Wang and T.-T. Tang, Adjustment of the antibacterial activity and biocompatibility of hydroxypropyl trimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium, Carbohydr. Polym., 2010, 81, 275–283 CrossRef CAS.
- Z.-X. Peng, B. Tu, Y. Shen, L. Du, L. Wang, S.-R. Guo and T.-T. Tang, Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface, Antimicrob. Agents Chemother., 2011, 55, 860–866 CrossRef CAS PubMed.
- H. Tan, Z. Peng, Q. Li, X. Xu, S. Guo and T. Tang, The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant Staphylococcus, Biomaterials, 2012, 33, 365–377 CrossRef CAS PubMed.
- H. Tan, S. Guo, S. Yang, X. Xu and T. Tang, Physical characterization and osteogenic activity of the quaternized chitosan-loaded PMMA bone cement, Acta Biomater., 2012, 8, 2166–2174 CrossRef CAS PubMed.
- P. Zhou, Y. Xia, J. Wang, C. Liang, L. Yu, W. Tang, S. Gu and S. Xu, Antibacterial properties and bioactivity of HACC-and HACC-Zein-modified mesoporous bioactive glass scaffolds, J. Mater. Chem. B, 2013, 1, 685–692 RSC.
- H.-Y. Li, Y.-Q. Tan, L. Zhang, Y.-X. Zhang, Y.-H. Song, Y. Ye and M.-S. Xia, Bio-filler from waste shellfish shell: preparation, characterization, and its effect on the mechanical properties on polypropylene composites, J. Hazard. Mater., 2012, 217, 256–262 CrossRef PubMed.
- J. R. Nefussi, M. Boy-Lefevre, H. Boulekbache and N. Forest, Mineralization in vitro of matrix formed by osteoblasts isolated by collagenase digestion, Differentiation, 1985, 29, 160–168 CrossRef CAS PubMed.
- C. Chua, The design of scaffolds for use in tissue engineering, Part I. Traditional factors, Tissue Eng., 2001, 7, 679–689 CrossRef PubMed.
- K. Whang, K. Healy, D. Elenz, E. Nam, D. Tsai, C. Thomas, G. Nuber, F. Glorieux, R. Travers and S. Sprague, Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture, Tissue Eng., 1999, 5, 35–51 CrossRef CAS PubMed.
- M. C. Wake, C. Patrick Jr and A. Mikos, Pore morphology effects on the fibrovascular tissue growth in porous polymer substrates, Cell Transplant., 1993, 3, 339–343 Search PubMed.
- J. Balmain, B. Hannoyer and E. Lopez, Fourier transform infrared spectroscopy (FTIR) and X-ray diffraction analyses of mineral and organic matrix during heating of mother of pearl (nacre) from the shell of the mollusc Pinctada maxima, J. Biomed. Mater. Res., 1999, 48, 749–754 CrossRef CAS PubMed.
- A. R. Shrivats, M. C. McDermott and J. O. Hollinger, Bone tissue engineering: state of the union, Drug Discovery Today, 2014, 19, 781–786 CrossRef CAS PubMed.
- J. Sowjanya, J. Singh, T. Mohita, S. Sarvanan, A. Moorthi, N. Srinivasan and N. Selvamurugan, Biocomposite scaffolds containing chitosan/alginate/nano-silica for bone tissue engineering, Colloids Surf., B, 2013, 109, 294–300 CrossRef CAS PubMed.
- N. Shanmugasundaram, P. Ravichandran, P. N. Reddy, N. Ramamurty, S. Pal and K. P. Rao, Collagen–chitosan polymeric scaffolds for the in vitro culture of human epidermoid carcinoma cells, Biomaterials, 2001, 22, 1943–1951 CrossRef CAS PubMed.
- V. D. Rani, K. Manzoor, D. Menon, N. Selvamurugan and S. V. Nair, The design of novel nanostructures on titanium by solution chemistry for an improved osteoblast response, Nanotechnology, 2009, 20, 195101 CrossRef PubMed.
- N. Saranya, S. Saravanan, A. Moorthi, B. Ramyakrishna and N. Selvamurugan, Enhanced osteoblast adhesion on polymeric nano-scaffolds for bone tissue engineering, J. Biomed. Nanotechnol., 2011, 7, 238–244 CrossRef CAS PubMed.
- C. Vitale-Brovarone, M. Miola, C. Balagna and E. Verné, 3D-glass-ceramic scaffolds with antibacterial properties for bone grafting, Chem. Eng. J., 2008, 137, 129–136 CrossRef CAS.
- S. P. Miguel, M. P. Ribeiro, H. Brancal, P. Coutinho and I. J. Correia, Thermoresponsive chitosan–agarose hydrogel for skin regeneration, Carbohydr. Polym., 2014, 111, 366–373 CrossRef CAS PubMed.
- L.-Y. Zheng and J.-F. Zhu, Study on antimicrobial activity of chitosan with different molecular weights, Carbohydr. Polym., 2003, 54, 527–530 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26805b |
| This journal is © The Royal Society of Chemistry 2016 |