Bis(perylene diimide) with DACH bridge as non-fullerene electron acceptor for organic solar cells†
Abstract
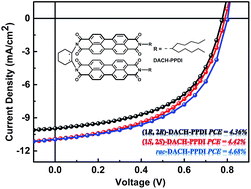
In this paper, we designed, synthesized, and characterized a set of non-fullerene small molecules based on bis(perylene diimide) with DACH bridge. Theoretical calculations make clear that the introduction of the DACH bridge into PPDI forms a U-shape framework, with pi–pi interactions between PDIs. We investigate the performances of non-fullerene solar cells comprising racemic and enantiomerically pure DACH-PPDIs as the electron acceptor and PTB7-Th as the electron donor. As a consequence, a power conversion efficiency (PCE) of 4.68% is achieved with inverted device architecture. Furthermore, the device behaviour, morphological feature and charge transport properties were also investigated. It is a potential way to design highly efficient non-fullerene organic solar cell by tuning the structure of PDI to reach highly efficient photovoltaic performances.

 Please wait while we load your content...
Please wait while we load your content...