Poly(3-hydroxybutyrate-co-valerate)/poly(3-thiophene ethyl acetate) blends as a electroactive biomaterial substrate for tissue engineering application
Abstract
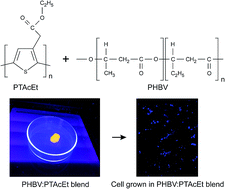
Polyblends of an electroactive biomaterial consisting of a biodegradable polyester – PHBV and a carboxylate polythiophene derivative, the poly(3-thiophene ethyl acetate) – PTAcEt have been prepared and applied as a substrate for tissue engineering. The biodegradable polymer was used as a matrix for polythiophene. Thermal, electrical, optical and morphological properties as well as viability and cell adhesion (in vitro assays) were investigated. Blend films exhibited high flexibility, resistance to handling and a semiconducting character, since they preserved the individual properties of each polymer. Besides, the incorporation of PTAcEt (up to 12%) has led to improvements in the thermal stability and in the crystallinity degree of the bioactive matrix. Qualitative morphological analysis showed irregular and porous surfaces; important features for growth and cell adhesion. Viability assays indicated that the polyblends promoted superior cell proliferation after 72 h exposure to mammalian cells when compared to a pure PHBV matrix. Therefore, PTAcEt showed low cytotoxicity allowing cell growth. The adhesion study revealed that the cells were able to adhere to the surface and proliferate inside the cavities of the substrate. The results indicate that the 88 : 12% (PHBV : PTAcEt) blend is a promising candidate for use as scaffold in the biomedical area.

 Please wait while we load your content...
Please wait while we load your content...