Mechanism for the formation and growth of carbonaceous spheres from sucrose by hydrothermal carbonization†
Abstract
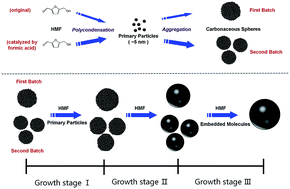
We report a new three-step mechanism for the formation and growth of carbonaceous spheres by hydrothermal carbonization of saccharides using sucrose as a precursor material. Carbonaceous spheres with small diameters and narrow size distribution were synthesized via a rapid heating route, and a notable phenomenon of a sudden drop in the mean diameter of the carbonaceous spheres at low concentration with the extension of time was observed. The morphology, chemical structure of carbonaceous spheres and the chemical composition of residual solutions were analysed by field emission scanning electron microscope (FESEM), Fourier transform infrared spectroscopy (FT-IR) and solution 13C nuclear magnetic resonance (NMR) respectively. Based on these results, evolution of solid products is clearly revealed. The formation contains two stages, and oversaturation of primary particles attributed to autocatalysis of fructose by the yielded acid (formic acid) results in the appearance of large amounts of carbonaceous spheres in the second stage of formation, which accounts for the sudden drop in mean diameter.

 Please wait while we load your content...
Please wait while we load your content...