The hydrogen sensing properties of Pt–Pd/reduced graphene oxide based sensor under different operating conditions
Yitian Peng*ab,
Jianxin Yea,
Lulu Zhenga and
Kun Zouab
aCollege of Mechanical Engineering, Donghua University, Shanghai 201620, China. E-mail: yitianpeng@dhu.edu.cn; Fax: +86 21 67874297; Tel: +86 21 67874297
bDonghua University, Engineering Research Center of Advanced Textile Machinery, Ministry of Education, Shanghai 201620, China
First published on 23rd February 2016
Abstract
Graphene is a two-dimensional nanomaterial with high specific surface area, excellent physical and chemical properties with great potential in sensor applications. Uniform nanocrystalline Pt–Pd nanoparticle-decorated reduced graphene oxide (Pt–Pd/RGO) has been synthesized via a one-step chemical reduction. Dielectrophoresis applied to Pt–Pd/RGO fabricated the sensor. The hydrogen sensing properties of Pt–Pd/RGO were studied by recording the resistance changes following exposure to hydrogen at different concentrations, temperatures, flow rate and with different carrier gases. The Pt–Pd/RGO sensor has a stable and repeatable response due to carrier donation and crystal lattice expansion over hydrogenation and dehydrogenation. The maximum response tends to increase with the increase of hydrogen concentration or decrease of operating temperature, but remained almost unaffected by the flow rate. The nitrogen as carrier gas possesses higher response and longer recovery time than air because oxygen participates in the reaction. These studies have potential for the development of novel H2 sensors.
1. Introduction
Hydrogen (H2) is one kind of clean and efficient combustible fuel superior to carbon-based fuels and holds considerable promise for extensive applications in the future. However, H2 is explosive in air over a concentration of 4 vol% and can hardly be detected since it is odorless and colorless. Therefore, developing sensors that can detect H2 gas under different operating conditions is essential. Various types of hydrogen sensors have been developed based on monitoring changes in electrical, optical, electrochemical, and mechanical signals.1–4 Among them, hydrogen sensors based on electrical resistance changes are promising due to the simplicity of their design and measurement. The sensing mechanism is mainly based on the fact that their electrical properties are extremely sensitive to charge transfer and chemical doping by H2. Nanomaterials such as metal nanowire, carbon nanotubes and graphene have been widely used as support materials in hydrogen sensors due to their unique properties.5–7Compared to one-dimensional sensing materials including nanowires and nanotubes, graphene has distinct advantages as a support material to detect hydrogen; its perfect two-dimensional structure with theoretical maximum specific surface area, high charge mobility and remarkable structural flexibility. Surface modification of graphene is regarded as a good approach to obtain sensitive H2 detection. Various catalytic hydrogen strategies attaching noble metal nanoparticles,8,9 metal oxide particles,10 and polymers11 have been developed to enhance the response because direct interaction of H2 with the unmodified graphene is too weak to cause a significant response. Metallic catalytic palladium (Pd) or platinum (Pt) nanoparticles coating the surface of graphene by chemical or physical functionalization, can enhance the detection response due to their high dissociation efficiency of molecular hydrogen to the more reactive atomic form. Cheap and facile reduced graphene oxide (RGO) has potential to be an alternative supporting material for hydrogen sensors. Catalytically active noble metals like Pt or Pd nanoparticles supported on high-surface-area RGO have also been investigated in previous work1,7,12 and exhibit good sensing responses.13–15 Pt nanoparticles have good adsorption and desorption properties on hydrogen, and Pd nanoparticles have high reactivity and durability. The combination of Pt and Pd nanoparticles could achieve synergistic effects to promote the performance of the H2 sensor. Moreover, Pt–Pd is known to show better hydrogenation properties in comparison to Pd.12 However, in contrast to modification with Pd or Pt individually, the chemical modification of RGO with both Pd and Pt nanoparticles remains largely unexplored.
In this paper, uniform crystalline Pt–Pd nanoparticles decorated RGO (Pt–Pd/RGO) has been synthesized via a one-step chemical reduction method. The Pt–Pd/RGO based sensor was fabricated on Au electrodes using dielectrophoresis (DEP) process. The hydrogen sensing properties of Pt–Pd/RGO based sensor were studied by recording the electrical resistance change when exposed to H2 at different concentrations, operating temperatures, flow velocities and carrier gases. Also, a possible mechanism was proposed to account for various H2 sensing characteristics.
2. Experimental
2.1 Preparation and characterization of the Pt–Pd/RGO
Graphite oxide was prepared by a modified Hummer’s method16 starting from graphite flakes of 50 μm size. The graphene oxide (GO) aqueous solution was obtained by ultrasonic exfoliation of graphite oxide in de-ionized (DI) water. GO was centrifuged and dried in a 60 °C vacuum drying oven for 2 days. The mixed solution of H2PtCl6·6H2O (2 ml, 0.05 M) and PdCl2 (2 ml, 0.05 M) was prepared by dissolving them in 200 ml aqueous solution. Subsequently, 10 mg of the as prepared GO powder was added followed by continuous stirring and ultrasonication. The mixed solution of NaBH4 (0.2 M) and NaOH (1 M) was prepared as a reducing agent of metal ion (Pd2+ and Pt2+) and GO. The reductant solution was added into the mixed solution under an ultrasonic environment. In order to reduce the GO completely, the resulting mixture was heated to 90 °C with stirring for another 4 h after a stable black suspension was formed. The product was repeatedly washed with DI water to remove the remaining reagents and centrifuged to get Pt–Pd/RGO. The Pd and Pt ions transform into crystals and are deposited on the surface of GO, accompanied by the reduction of GO to RGO. The black solid was dried overnight in a vacuum at 60 °C and the powder was obtained.The morphology and structure of the prepared Pt–Pd/RGO was characterized by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Dispersed Pt–Pd/RGO in ethanol was dropped on a silicon substrate for SEM characterization. The Energy Dispersive Spectrometer (EDS) in SEM was used to analyze the composition of Pt–Pd/RGO. The Pt–Pd/RGO ethanol suspension was dropped on a mesh copper grid for TEM characterization. The crystal structure of the Pt–Pd/RGO was characterized by powder X-ray diffraction (XRD) using Cu–K radiation with a Bruker D8 Advance.
2.2 Fabrication of the Pt–Pd/RGO based sensor
The Pt–Pd/RGO powders (2 mg) were ultrasonically dispersed in 10 ml ethanol for 12 min to produce a stable suspension. The Au (90 nm)/Ti (5 nm) electrode array for DEP assembly was fabricated via a typical lift-off process. Fig. 1(a) displays the optical microscope image of the triangle Au electrodes on silicon substrate with 300 nm of SiO2. The electrode array contains five pairs of electrodes numbered from 01 to 05. The gap size of electrodes is 2 μm. Well-dispersed Pt–Pd/RGO suspension was deposited onto triangular Au electrodes using DEP of alternating current with 10 V peak-to-peak sinusoidal wave and 1 MHz frequency. Fig. 1(b) shows the schematic diagram of the DEP assembly. The Pt–Pd/RGO can be easily and precisely positioned on the desired electrodes. Fig. 1(c) and (d) show the schematic and optical microscope images of a uniformly distributed Pt–Pd/RGO deposited on the electrodes after DEP assembly. The thickness of the Pt–Pd/RGO deposited on the Au electrodes was controlled by adjusting the deposition time and suspension concentration. The atomic force microscopy images of the Pt–Pd/RGO thin film are provided in the inset image in Fig. 1(d). It can be seen that the thickness of the film is about 200 nm.2.3 Hydrogen sensing measurements of the Pt–Pd/RGO based sensor
Hydrogen sensing measurements of the Pt–Pd/RGO based sensor were carried out in a dynamic gas flow glass chamber of volume 150 ml. The schematic diagram of the setup for hydrogen sensing measurements is shown in Fig. 2. Three mass flow controllers (MFCs) were connected to the chamber to supply gas and form a single output. The ratio and flow velocity of gas, including the H2 and carrier gas, was accurately controlled by the MFCs. The H2 in the carrier gas was injected into the chamber through a mixer. Two signal wires feed through the end of the chamber to connect the Pt–Pd/RGO based sensor on a self-made probe station. The probe station was made from two copper probes and substrate holder to connect the Pt–Pd/RGO based sensor with an outer electrical circuit. The operating temperatures can be adjusted by a compact programmable heater integrated on the substrate holder. The hydrogen sensing characteristics of the Pt–Pd/RGO based sensor were measured by using a two-probe station with a Keithley 2612B Source Meter to record the resistance change. The computer was used to adjust the MFCs and collect the measurement data automatically.Before taking the gas sensing measurements, a continuous stream of carrier gas was injected to clean the chamber for 5 min. The sensing experiments were carried out when different concentrations of H2, of specific ratio, were injected into the chamber with the carrier gas. The sensors were exposed to a H2 gas pulse sequence of different concentrations in carrier gas at room temperature for measurement. The gas mixture was delivered at a constant flow rate.
3. Results and discussion
3.1 Characterization of Pt–Pd/RGO
Fig. 3(a) shows the SEM image of Pt–Pd/RGO and it can be seen that uniformly dispersed Pt–Pd nanoparticles are deposited on the surface of RGO. The EDS analysis of Pt–Pd/RGO in Fig. 3(b) shows C, O, Si, Pt and Pd. It suggests that both the Pt and Pd particles have been reduced from the mixed solution, and no impurity is introduced during the reduction process. The inset table shows detailed percentages of different elements of the Pt–Pd/RGO on silicon substrate. The atomic percentages of Pt and Pd were 4.10% and 3.08%, respectively. The strong silicon peak comes from silicon substrates.Fig. 3(c) clearly shows the uniformly well-dispersed Pt and Pd nanoparticles deposited on the RGO. The sizes of the Pt–Pd nanoparticles range from ∼3 nm to ∼8 nm. The crystalline structure of Pt–Pd/RGO was further characterized by high magnification TEM (inset of Fig. 3(c)). It can be seen the Pt and Pd nanoparticles were exclusively crystalline from the fringe pattern of the entire high-resolution image.
XRD pattern of the Pt–Pd/RGO is given in Fig. 3(d). Four strong diffraction peaks at 2θ values of 39.9°, 46.4°, 67.6°, and 81.4° agree well with the (111), (200), (220) and (311) crystalline planes of Pt–Pd nanoparticles, respectively. The 2θ values at the peaks of Pt–Pd nanoparticles are just between those of pure Pt and Pd nanoparticles, indicating that the crystalline Pt–Pd nanoparticles have been reduced and deposited on the surface of RGO. The d-spacing at the strongest (111) peak of Pt–Pd is 2.253 Å.
3.2 Hydrogen sensing properties of the Pt–Pd/RGO based sensor
The I–V characteristics of the RGO with resistance of 9.6 MΩ, and Pt–Pd/RGO with resistance of 1.06 MΩ, were measured when exposed to H2 at different concentrations in air. Fig. 4 shows the I–V characteristics of the Au contacts when exposed to H2 under 300 sccm gas flow rate at room temperature. The resistance of pure RGO shows negligible change when exposed to H2 with concentrations from 1000 ppm to 8000 ppm in air; despite the residue defects and functional groups present on the surface of RGO acting as high-energy sites for H2, the overall interaction is negligible. Evident decreases in resistance were observed when the Pt–Pd/RGO based sensor was exposed to H2 in air. Also, the resistance tends to decrease with increasing H2 concentration. It can be deduced that Pt–Pd nanoparticles on the surface of RGO play a critical role on the resistance decreases. | ||
| Fig. 4 I–V characteristics of the RGO (a), and Pt–Pd/RGO (b), exposed to H2 at different concentrations in air. | ||
The resistance decrease of Pt–Pd/RGO based sensors can be attributed to the well-known adsorption and desorption8 of atomic hydrogen dissociated from molecules by the catalytic effect of Pt and Pd, which results in the formation of platinum hydrides (Pt/H)17 and palladium hydrides (Pd/H).18 The formation of Pt/H and Pd/H changes the physical and geometrical properties of the nanoparticles. The pristine graphene shows a maximum resistance corresponding to carrier concentration (Dirac point). The RGO coated with Pt–Pd nanoparticles could behave like an N-type sensor after oxidation, reduction and dispersion treatment at room temperature. The Pt and Pd hydrides lower the work function of Pt and Pd nanoparticles resulting in electron donation from the metal hydride to the RGO. The resultant increase of carrier concentration of the Pt–Pd/RGO based sensor in the absence of H2 improved the electrical conductivity. Furthermore, the individual Pt and Pd nanoparticles swell due to lattice expansion during Pt–Pd hydride formation and narrow the distance of interparticle gaps.19,20 The reduction gaps between neighboring nanoparticles lead to a decrease in the distance of the conducting route and result in an improvement of electrical conductivity. So, atomic hydrogen combines with Pt and Pd to form Pt/H and Pd/H, improving the electrical conductivity. The change of electrical conductivity tends to increase as the hydrogen concentration increases.
The influence of the recovery to the sensor response is determined by recording the resistance decrease when exposed to hydrogen of different concentrations. In order to evaluate the performance, the response of the Pt–Pd/RGO based sensor was defined as:1,7,21
| Response = ΔR/R0 × 100% = (R0 − R)/R0 × 100%, |
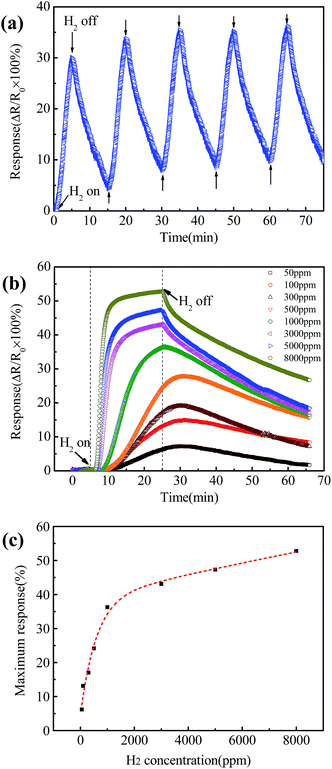
Fig. 5(a) shows the response of the Pt–Pd/RGO based sensor as a function of time exposed to a hydrogen concentration of 5000 ppm for 5 min on and 10 min off alternately in air at a flow rate of 300 sccm. It can be observed that the ΔR/R0 increased quickly when the H2 flowed through the chamber. The ΔR/R0 of the sensor recovered quickly when the H2 was stopped and only pure air was introduced. More than 95% of the original value was recovered only after ten min off of H2. The sensor has a stable response over repeated cycles of hydrogenation and dehydrogenation. Fig. 5(b) shows the response change with single exposures to H2 at different concentrations ranging from 50 to 8000 ppm in air for 20 min. The response increases quickly during the first 12 min exposure, then increases slowly due to saturation.20 Also the response at high concentrations is faster than low concentrations. The response decreases when H2 is shut off and only pure air filled the glass chamber, the curve shows a slower and weaker response as the hydrogen concentration decreases.
Fig. 5(c) plots the maximum response extracted from Fig. 5(b) as a function of the hydrogen concentration ranging from 50 to 8000 ppm at a flow rate of 300 sccm. It can be seen that the maximum response increases from ∼6% to ∼52% when the hydrogen concentration increases from 50 ppm to 8000 ppm. There is a monotonic relationship between the maximum response and H2 concentration. The maximum response increases fast at low concentrations, then slow at high concentrations. The schematic illustration of the Pt–Pd/RGO preparation and H2 sensing is shown in Fig. 6. Adsorption, including physisorption and chemisorption, is introduced. During physisorption, hydrogen is adsorbed on the surface of Pt–Pd nanoparticles as molecules or atoms by weak van der Waals forces. The chemisorption enables hydrogen atoms to enter the lattice of the metal forming metal hydrides with strong covalent bonds.8 Chemisorption needs more time than physisorption because more energy is needed to form metal hydride bonds. Hydrogen at high concentration could provide plenty of available molecules, and more adsorbed H2 on Pt and Pd nanoparticles will be transformed into Pt/H and Pd/H, respectively. This can be related to the extra time that is needed at low concentrations for the few available hydrogen molecules to find Pt and Pd adsorption sites.1 The quick recovery of the sensor could be attributed to the physical desorption as it is much easier than chemical desorption. Lattice contraction reduces the size of the crystalline Pt–Pd nanoparticles during dehydrogenation which also contributes to the recovery process. The improved response at higher concentrations is attributed to the increased coverage of hydrogen atoms on the Pt–Pd/RGO surface. It is not surprising that the response time is quicker at high concentrations because the H2 absorbed easily on Pt and Pd. The effect of H2 concentration reduced as the concentration increases due to saturation.
3.3 Effects of temperature, flow rate and carrier gas on hydrogen sensing properties
To investigate the effect of temperature on H2 sensing properties, electrical conductivity measurements of the Pt–Pd/RGO based sensor were carried out when exposed to 3000 ppm H2 in air at different operating temperatures. Fig. 7(a) shows the response of the Pt–Pd/RGO based sensor as a function of time when the temperature varied from 25 to 65 °C. The response increases quickly when exposed to H2 under all 5 temperatures, then recovers slowly. During the sensor recovery section, it is interesting that the recovery is slow when the temperature is below 45 °C and fast above 45 °C. Fig. 7(b) shows the temperature dependence of maximum response of the Pt–Pd/RGO based sensor when exposed to 3000 ppm H2 in air. It is obvious that the maximum response decreases in magnitude as the temperature increases at a constant H2 concentration. From the curve fit, there exists a linear relationship between the maximum response and temperature. The reason for the slower response at high temperature may come from the diffusion of the dissociatively adsorbed hydrogen away from the surface. Also, the maximum response decreases as the temperature increases because high temperature impedes the physisorption process. | ||
| Fig. 7 (a) Response of the sensor exposed to 3000 ppm H2 in air as a function of time at 5 different temperatures. (b) Maximum response as a function of temperature. | ||
The flow rate of the carrier air gas is also taken into account. Fig. 8(a) shows the response of the Pt–Pd/RGO based sensor upon exposure to a permanent hydrogen concentration of 3000 ppm in air at different flow rates. The response increases quickly as the flow rates increases from 300 to 1200 sccm. Although increase velocities were different, the final value of response increased to the same level. Fig. 8(b) also plots the dependence of maximum response on the flow rate. The maximum response varied a little bit with four different flow rates. It can be concluded that the flow rate of the carrier gas has an insignificant effect on the maximum response. It is reasonable that as the number of H2 molecules per unit volume does not change when the concentration keeps the same, the number of Pt or Pd hydrides exposed to H2 remains the same. The recovery is faster at higher flow rate than lower flow rate as the adsorbed H2 molecules are more easily removed.
 | ||
| Fig. 8 (a) Response as a function of time at different flow rates. (b) Maximum response versus flow rate of carrier gas. | ||
Oxygen makes up 21% of air and the majority of the remaining gas is nitrogen. The role of oxygen in hydrogen sensing was studied using two types of carrier gas: N2 and air. Fig. 9(a) shows the time dependence of the response when exposed to 1000 ppm H2 with different carrier gases including N2, air and N2 switched back to air as time proceeds for 35 min. The response increases quickly and recovers slowly when exposed to 1000 ppm H2 and N2 compared with air. The maximum response of the Pt–Pd/RGO based sensor under N2 (∼40%) is almost four times larger than in air (∼10%). As the carrier gas is switched from N2 back to air after 35 min, the sensor recovers quickly. It can be concluded that the oxygen molecules in air reduce the response but promote the recovery of the sensor. These large differences in response and recovery changes imply that the oxygen molecules in air take part in the reactions between Pt–Pd nanoparticles and H2.
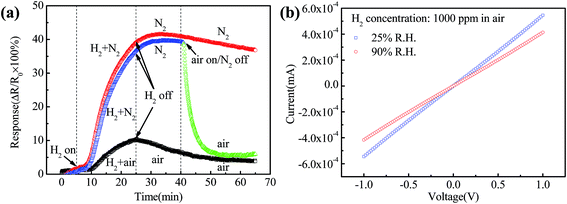 | ||
| Fig. 9 (a) Response of the sensor exposure to 1000 ppm hydrogen in carrier gas. (b) I–V characteristic of the sensor exposed to 1000 ppm hydrogen in air under two different relative humidities. | ||
The reactions of oxygen molecules between hydrogen and Pt/Pd nanoparticles in the adsorption and desorption processes can be described as follows:18,22
| Adsorption: H2 ↔ 2[H]; 6[H] + Pt + Pd + O2 ↔ Pt/H + Pd/H + 2H2O | (1) |
| Desorption: O2 + Pt/H + Pd/H + 2[H] → Pt + Pd + 2H2O | (2) |
During the hydrogen adsorption process, the oxygen molecules combine with dispersed hydrogen and Pt–Pd nanoparticles to form Pt/H and Pd/H. The hydrogen atoms could react with not only Pt–Pd nanoparticles but also oxygen molecules. When nitrogen serves as the hydrogen carrier gas, no reaction happens between hydrogen and the carrier gas so there exists more hydrogen atoms to react with Pt and Pd nanoparticles causing the resistance to decrease greatly.
The oxygen molecules react with hydrogen and reduced hydrogen concentration. The decreased hydrogen concentration could explain why the hydrogen response in air is obviously lower than that of nitrogen. For desorption, the effective oxygen molecules are proposed to react with Pt/H and Pd/H to form Pt–Pd nanoparticles and combine with dissociated hydrogen atoms to form water molecules. The resistance recovery is expected mainly to arise from the reaction of the dissolved hydrogen with oxygen. These reactions consume the residual hydrogen molecules and enable the resistance to recover to the initial level quickly. The sensor recovered slowly as the nitrogen contains no oxygen. Also, the water produced in the reaction reduces the adsorption energy of Pt–Pd nanoparticles adhering to the surface of RGO, which increases the resistance rapidly. In order to study the effect of water vapor on the resistance, Fig. 9(b) displays the I–V characteristics of the Pt–Pd/RGO based sensor exposure to 1000 pm H2 in air under 25% and 90% R.H. at room temperature. The conductivity did decrease when the relative humidity switched from 25% to 90% R.H. The Pt–Pd/RGO based sensor displays a pronounced increase in resistance upon introduction of water, followed by a decrease upon exposure to H2. So the relative humidity plays a role in restoring the electrical response. The oxygen in air plays an important role during H2 adsorption and desorption determining the mechanisms for electron transport and gas sensing.
4. Conclusion
The Pt–Pd/RGO with uniform nanocrystalline Pt–Pd nanoparticles coating RGO was synthesized via a one-step chemical reduction route. The Pt–Pd/RGO based sensors were fabricated using a fast and efficient DEP assembly process. The Pt–Pd/RGO based sensors had a repeatable and stable response to H2 due to carrier donation and crystal lattice expansion from metal hydride formation. The temperature reduces the response, but increased hydrogen concentration improves it. The flow rate of the carrier gas has negligible impact on the response. High response and slow recovery can be obtained when using nitrogen replaced by air as the carrier gas due to the involvement of oxygen. The Pt–Pd/RGO based sensors could serve as facile hydrogen sensors with high-performance including excellent response and recovery under various environments.Acknowledgements
This work is supported by the Fundamental Research Funds for the Central Universities (Grant No. 2232015A3-05) and Shanghai Natural Science Foundation (Grant No. 15ZR1400700), the New Century Training Program Foundation for the Talents by the State Education Commission (Grant No. NCET-11-0095), and the Natural Science Foundation of China (Grant No. 51002030).References
- J. L. Johnson, A. Behnam, S. J. Pearton and A. Ural, Hydrogen Sensing Using Pd-Functionalized Multi-Layer Graphene Nanoribbon Networks, Adv. Mater., 2010, 22(43), 4877–4880 CrossRef CAS PubMed.
- D. Monzon-Hernandez, D. Luna-Moreno and D. Martinez-Escobar, Fast response fiber optic hydrogen sensor based on palladium and gold nano-layers, Sens. Actuators, B, 2009, 136(2), 562–566 CrossRef CAS.
- R. S. Sundaram, C. Gomez-Navarro, K. Balasubramanian, M. Burghard and K. Kern, Electrochemical modification of graphene, Adv. Mater., 2008, 20(16), 3050–3053 CrossRef CAS.
- S. Mubeen, T. Zhang, B. Yoo, M. A. Deshusses and N. V. Myung, Palladium nanoparticles decorated single-walled carbon nanotube hydrogen sensor, J. Phys. Chem. C, 2007, 111(17), 6321–6327 CAS.
- A. Z. Sadek, W. Wlodarski, K. Kalantar-Zadeh, C. Baker and R. B. Kaner, Doped and dedoped polyaniline nanofiber based conductometric hydrogen gas sensor, Sens. Actuators, A, 2007, 139(1–2), 53–57 CrossRef CAS.
- Y. G. Sun and H. H. Wang, Electrodeposition of Pd nanoparticles on single-walled carbon nanotubes for flexible hydrogen sensors, Appl. Phys. Lett., 2007, 90(21), 213107 CrossRef.
- Z. Y. Zhang, Q. Z. Xue, Y. G. Du, C. C. Ling and W. Xing, Highly enhanced sensitivity of hydrogen sensors using novel palladium-decorated graphene nanoribbon film/SiO2/Si structures, J. Mater. Chem. A, 2014, 2(38), 15931–15937 CAS.
- A. Kaniyoor, R. I. Jafri, T. Arockiadoss and S. Ramaprabhu, Nanostructured Pt decorated graphene and multi walled carbon nanotube based room temperature hydrogen gas sensor, Nanoscale, 2009, 1(3), 382–386 RSC.
- L. C. Tien, P. W. Sadik, D. P. Norton, L. F. Voss, S. J. Pearton, H. T. Wang, B. S. Kang, F. Ren, J. Jun and J. Lin, Hydrogen sensing at room temperature with Pt-coated ZnO thin films and nanorods, Appl. Phys. Lett., 2005, 87(22), 222106 CrossRef.
- S. F. Bamsaoud, S. B. Rane, R. N. Karekar and R. C. Aiyer, Nano particulate SnO2 based resistive films as a hydrogen and acetone vapour sensor, Sens. Actuators, B, 2011, 153(2), 382–391 CrossRef CAS.
- S. Srivastava, S. S. Sharma, S. Agrawal, S. Kumar, M. Singh and Y. K. Vijay, Study of chemiresistor type CNT doped polyaniline gas sensor, Synth. Met., 2010, 160(5–6), 529–534 CrossRef CAS.
- R. Kumar, D. Varandani, B. R. Mehta, V. N. Singh, Z. H. Wen, X. L. Feng and K. Mullen, Fast response and recovery of hydrogen sensing in Pd-Pt nanoparticle-graphene composite layers, Nanotechnology, 2011, 22(27), 275719 CrossRef PubMed.
- P. A. Russo, N. Donato, S. G. Leonardi, S. Baek, D. E. Conte, G. Neri and N. Pinna, Room-Temperature Hydrogen Sensing with Heteronanostructures Based on Reduced Graphene Oxide and Tin Oxide, Angew. Chem., Int. Ed., 2012, 51(44), 11053–11057 CrossRef CAS PubMed.
- A. Esfandiar, S. Ghasemi, A. Irajizad, O. Akhavan and M. R. Gholami, The decoration of TiO2/reduced graphene oxide by Pd and Pt nanoparticles for hydrogen gas sensing, Int. J. Hydrogen Energy, 2012, 37(20), 15423–15432 CrossRef CAS.
- K. Anand, O. Singh, M. P. Singh, J. Kaur and R. C. Singh, Hydrogen sensor based on graphene/ZnO nanocomposite, Sens. Actuators, B, 2014, 195, 409–415 CrossRef CAS.
- S. W. Hummers and R. E. Offeman, Preparation of Graphitic Oxide, J. Am. Chem. Soc., 1958, 80(6), 1339 CrossRef.
- M. A. Uddin, A. K. Singh, T. S. Sudarshan and G. Koley, Functionalized graphene/silicon chemidiode H2 sensor with tunable sensitivity, Nanotechnology, 2014, 25(12), 125501 CrossRef PubMed.
- P. A. Pandey, N. R. Wilson and J. A. Covington, Pd-doped reduced graphene oxide sensing films for H-2 detection, Sens. Actuators, B, 2013, 183, 478–487 CrossRef CAS.
- F. Favier, E. C. Walter, M. P. Zach, T. Benter and R. M. Penner, Hydrogen sensors and switches from electrodeposited palladium mesowire arrays, Science, 2001, 293(5538), 2227–2231 CrossRef CAS PubMed.
- M. Khanuja, S. Kala, B. R. Mehta and F. E. Kruis, Concentration-specific hydrogen sensing behavior in monosized Pd nanoparticle layers, Nanotechnology, 2009, 20(1), 015502 CrossRef PubMed.
- J. Wang, Y. Kwak, I. Y. Lee, S. Maeng and G. H. Kim, Highly responsive hydrogen gas sensing by partially reduced graphite oxide thin films at room temperature, Carbon, 2012, 50(11), 4061–4067 CrossRef CAS.
- Y. G. Sun, H. H. Wang and M. G. Xia, Single-walled carbon nanotubes modified with Pd nanoparticles: unique building blocks for high-performance, flexible hydrogen sensors, J. Phys. Chem. C, 2008, 112(4), 1250–1259 CAS.
| This journal is © The Royal Society of Chemistry 2016 |