Cadmium and lead accumulation and low-molecular-weight organic acids secreted by roots in an intercropping of a cadmium accumulator Sonchus asper L. with Vicia faba L.
Abstract
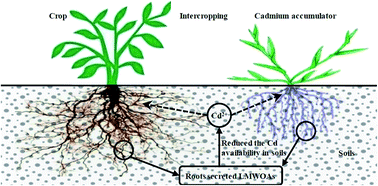
Sonchus asper L. and Vicia faba L. are a local cadmium (Cd) accumulator and a main winter crop, respectively, found in the Huize lead–zinc mining area in Yunnan Province, Southwest China. The biomass and low-molecular-weight organic acids (LMWOAs) secreted by the roots of these plants, Cd and lead (Pb) contents and their accumulation in a S. asper monoculture, V. faba monoculture and S. asper/V. faba intercrop were investigated in a field experiment at 35, 80 and 180 d after planting. The results showed that (1) intercropping had no notable influences on plant biomass and grain yields of V. faba but led to a significant increase in the amount of stem and leaf biomass of S. asper at 180 d after planting. (2) The major LMWOAs secreted by the roots of both V. faba and S. asper were oxalic acid, tartaric acid and citric acid. Intercropping resulted in an increase and decrease in the LMWOA contents secreted by V. faba and S. asper roots, respectively. (3) Along with plant growth, the available Cd content decreased and the available Pb contents did not exhibit obvious changes in the soil samples of a V. faba monoculture. The amount of available Cd and Pb both increased in the soil of the S. asper monoculture, but decreased in that of the S. asper/V. faba intercrop. (4) Intercropping resulted in a decrease in the contents and accumulation of Cd and Pb in V. faba plants, but an increase in both the contents and accumulation of Cd and Pb in S. asper plants. Moreover, intercropping enhanced the enrichment and translation coefficients of Cd for S. asper. The remediation efficiency was the highest at 180 d after planting. (5) There were significant negative correlations between the contents of citric acid, malic acid (secreted by V. faba roots), oxalic acid and tartaric acid (secreted by S. asper roots) and the available Cd content in the soil samples. In addition, there was a significant positive correlation between the available Cd content in the soil and the Cd contents in the roots and grains of V. faba. Intercropping reduced the Cd contents in the plants and grains of V. faba and was closely related to the decrease in the available Cd content in the soil samples, which was mediated by plant roots that secreted LMWOAs.

 Please wait while we load your content...
Please wait while we load your content...