Optimization of a broth conductivity controlling strategy directed by an online viable biomass sensor for enhancing Taxus cell growth rate and Taxol productivity
Ze-Jian Wangab,
Wei Zhanga,
Jian-Wen Zhanga,
Mei-Jin Guoa and
Ying-ping Zhuang*a
aState Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, P.O. Box 329, 130 Meilong Road, Shanghai 200237, China. E-mail: ypzhuang@ecust.edu.cn; Fax: +86-21-64253702
bDepartment of Biotechnology, Delft University of Technology, Julianalaan 67, 2628BC Delft, The Netherlands
First published on 13th April 2016
Abstract
Online process control is always an important and challenging technique for anti-cancer Taxol (paclitaxel) production by a plant cell suspension culture due to its slow growth rate, variability and intense sensitivity to environmental factors. In this work, we investigated the effects of a constant broth conductivity controlling strategy directed by an online viable biomass sensor on Taxol productivity of Taxus chinensis var. mairei in suspension cultivation. The viable cell capacitance was effectively used for detecting Taxus cell concentration online and for optimal feeding of the elicitor to stimulate Taxol production. An increase in conductivity has a negative effect on cell growth and elongation. With the optimal broth conductivity constantly controlled at 2.6 mS cm−1 and methyl jasmonate at 6 μmol g−1 cell, the Taxus cell growth rate and Taxol production reached their highest value at 0.87 g d−1 and 76.2 mg L−1 at 8 days after methyl jasmonate addition, and productivity reached its highest at 850 μg per g DCW per d, which was much higher than that under the higher conductivity conditions. Therefore, a substrate real-time feedback control strategy based on capacitance and conductivity must be an effective method for enhancing Taxol productivity and can be used for the scale up of a plant cell suspension culture.
1. Introduction
Taxol (paclitaxel) is isolated from yew (Taxus brevifolia) bark extracts and generally used as an anti-cancer drug due to its inhibitory effect on cell proliferation through binding to the microtubule surface.1,2 The content of Taxol in nature is very small. One has to sacrifice three or four 150–200 year old trees to extract sufficient Taxol for a cancer patient. Recently, a growing market for Taxol has made it an urgent demand for enhancing production.3,4 Taxol can be produced through four general approaches: complete chemical synthesis, semi-synthesis from the natural precursor 10-deacetylbaccatin III, large scale fermentation using fungi or bacteria, and in situ production using plant cell culture.4,5 For the complete synthesis of paclitaxel, two different routes were developed in 1994, but there were severe limitations due to the complex reactions involved (over 40 reactions were required), harsh solvents and low yield. Even though the natural precursor was renewable in the semi-synthesis method, limitations made both approaches economically and environmentally unfavorable.6 Research on the discovered entophytic Taxol-producing fungus in the Taxus genus demonstrated only low yields and high cost for Taxol production.7 In contrast, a plant cell suspension culture is a more sustainable way for producing Taxol under more easily controlled conditions. This Taxol production method has been licensed by Bristol-Myers Squibb to Phyton Biotech Inc. (Fort Worth, TX), and employs a large-scale fermentor with a capacity up to 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 L.8 Furthermore, many steps of the biosynthetic pathway have been elucidated through the isolation of genes and enzymes from Taxus tissues and cultured cells.5 However, the application of this technology is still limited by the slow growth rates, variability, and difficulties associated with scaling up production.9 Thus, much research has focused on optimizing culture conditions, screening high yielding cell callus, and the induction of secondary metabolite pathways by elicitors, in flask or solid culture mediums.1,5,10,11
000 L.8 Furthermore, many steps of the biosynthetic pathway have been elucidated through the isolation of genes and enzymes from Taxus tissues and cultured cells.5 However, the application of this technology is still limited by the slow growth rates, variability, and difficulties associated with scaling up production.9 Thus, much research has focused on optimizing culture conditions, screening high yielding cell callus, and the induction of secondary metabolite pathways by elicitors, in flask or solid culture mediums.1,5,10,11
A stirred tank bioreactor (STR) and pneumatically agitated bioreactor (air-lift and bubble column bioreactor) are the most widely used equipment for large-scale fermentation processes. Compared with pneumatically agitated bioreactor, STR has advantages such as easy scale-up, good fluid mixing and oxygen transfer ability, alternative impellers, and easy compliance with current good manufacturing practice requirements.12 However, STR always takes place in a high shear environment, which is not particularly suitable for plant cells as they aggregate and are very sensitive to the external environment. Even changing culture conditions from a flask to another type of reactor may lead to browning or blackening of the culture, which is frequently observed when culturing woody plant tissues; the discoloration is caused by the oxidation of phenols after cellular disorganization.13 The general solution to reducing shear stress intensity is to decrease the agitation speed of the impeller. The scale-up of a Taxus suspension from a flask to STR is also affected by factors such as the vitality of cells, process parameters (e.g., oxygen levels, agitation)14 and the need to supplement nutrients such as sugar and major elements.
The accumulation of biomass in STR is crucial for the yield of Taxol. Currently, the most widely used method for biomass measurement is dry cell weight (DCW), which is a conventional off-line method, but it can hardly be monitored in real time. In the present study, the biomass monitor,15 which can simultaneously evaluate viable cells and conductivity on-line, was used in a 5 L stirred tank bioreactor. Not only the growth status of Taxus cells could be measured, but also the concentrations of nutrients in the system, which were characterized by conductivity measurements. Normally, the conductivity of fresh culture media is 3.9–4.0 mS cm−1, and it is necessary to subculture or feed additional medium when the value declines to about 2.3–2.4 mS cm−1.
Our research investigated the influence of conductivity on plant cell growth rate and the productivity of secondary metabolism through the utilization of a biomass electrode. The extracellular amino acid was also measured to evaluate the variation of metabolism at the cellular level under different controlling strategies. Further aims were to elucidate the variables that influence fluctuations in secondary metabolite levels and to provide some guidance for the industrial production of Taxol.
2. Materials and methods
2.1 Plant material and culture conditions
The cell culture of Taxus chinensis var. mairei, originally obtained from Guangdong Kelun Pharmaceutical Company, was stored at the State Key Laboratory of Bioreactor Engineering, East China University of Science and Technology. Cells were cultivated in Gamborg’s B5 medium, and callus cultures were transferred every 24 days to a fresh solidified sterile medium. Yellowish-white pigmented cell aggregates were preferentially selected. Cell suspension cultures were established by transferring cell aggregates into 100 mL of B5 medium in 250 mL Erlenmeyer flasks, which were continuously agitated on a rotary shaker at 110 rpm. The cell cultures were transferred to new media every 14 days. Cultures were maintained at 25 ± 0.5 °C in the dark. The inoculum size was about 100 g of fresh weight per liter of medium. A bubble-column bioreactor was also used as a cultivated reactor to obtain a large amount of plant cells. Compressed air was filter-sterilized and fed to the reactor through a sparger at a rate of 0.2–0.3 vvm.2.2 Preparation of the feeding solution
The feeding solution contained methyl jasmonate (MeJA), Ag+ at a final concentration of 50.0 μM, 500 g L−1 sucrose and Major Element (ME) solution (20 × ME solution contained 50.0 g L−1 KNO3; 5.0 g L−1 MgSO4·7H2O, 3.0 g L−1 CaCl2·2H2O, 2.7 g L−1 (NH4)2SO4 and 3.0 g L−1 NaH2PO4·H2O). MeJA was dissolved in twain (at a concentration of 10 mM) and sterilized by membrane filtration for subsequent use as an elicitor in the production stage. The sucrose concentration of the broth was controlled at 30 ± 2 g L−1 throughout the culture process by adding pre-sterilized concentrated sucrose solution. The continuous feed medium (FM) contained ME (80% v/v) and B5 (20% v/v).2.3 Shake flask and stirred tank reactor experiments
Two stages of fermentation were carried out in the shake flask and stirred bank reactors, which involved the growth and production stages. The first stage was performed in a column reactor to obtain an abundant homogeneous cell broth. The second stage was conducted in the shake flasks and STR with elicitor stimulation. Fed-batch fermentation was carried out in a 500 mL shake flask with a 200 mL working volume. When the culture in the column reactor approached the subculture time, 100 mL of broth was incubated into 5 groups of shake flasks, with 100 mL of fresh B5 medium in each for further investigations. The cultivation conditions were the same as documented in subsection 2.2 above. Different methyl jasmonate (MeJA) concentrations (1.0, 3.0, 6.0, 9.0 and 12.0 μmol g−1 DCW) were added to investigate the influence of MeJA on the cell growth and yield. In 4 parallel 5 L STR fermentations, 1.5 L of broth from the bubble column reactor were mixed with the same volume of fresh medium to study the influence of conductivity on the cell growth and yield. The stirred tank reactor was equipped with a 3-bladed impeller, temperature probe, dissolved oxygen probe, and a radio frequency impedance spectroscopy biomass probe (Hamilton Instruments Ltd., Switzerland) was used to detect and monitor the experimental process. The temperature was maintained at 25 °C and the rotation speed and ventilator rate were initially set at 80 rpm and 1.5 L min−1, respectively. These values were adjusted according to the range of dissolved oxygen detected, which was controlled between 30% and 40%. The inlet and exhaust gas ingredients were analyzed by a mass spectrometer (MAX300-LG, Extrel). The CO2 evolution rate (CER) value was calculated and collected online.162.4 Determination of dry cell weight, residual sugar and cell viability
Dry cell weight (DCW): samples were filtered through a 100 mesh cloth and dried to a constant weight in a thermotank at 60 °C (Yiheng Scientific Instrument Co. Ltd, Shanghai, China). The residual sugar concentration was measured using the Anthrone method17 and cell viability was determined by the 2,3,5-triphenyl tetrazolium chloride (TTC) reduction method.18 300 mg of freshly weighed cells were placed in a centrifuge tube with 3.5 mL of 23.9 mM TTC solution and 3.5 mL of potassium phosphate buffer. After setting the mixture at 25 °C for 12 h in the dark, the supernatant was carefully removed with a pipette and the red cells washed in potassium phosphate buffer 3 times. Next, 5 mL of ethanol was added to each tube and the cells were pulverized in a 60 °C water bath for 30 min. Subsequently, the absorbance of the supernatant at 485 nm was determined using a spectrophotometer. All experiments were performed in triplicate.2.5 Evaluation of Taxol content and extracellular amino acid
Taxol (paclitaxel): methanol (20 mL) was added to 5 mL aliquots of the cell culture, which were then sonicated for 30 min at room temperature to extract Taxol. Samples were then stored at −20 °C for subsequent HPLC quantification. The Taxol content was measured using an external standard method and compared with pure Taxol obtained from Guangdong Kelun Pharmaceutical Company. Standard Taxol solutions of 0 (solvent only), 5, 10, 20, 40, 60 and 80 mg L−1 of Taxol were used to generate the calibration curve. A reverse-phase column (WondaSil™, C18, 5 μm, 250 × 4.6 mm) was used, which was operated at a temperature of 35 °C. The mobile phase consisted of acetonitrile (eluent A) and ultrapure water (eluent B). A multistep gradient was used for all separations, with an initial injection volume of 10 μL and a flow rate of 1.5 mL min−1. The multistep gradient was as follows: 0 min, 60% (v/v) B; 0–7 min, 60–40% B; 7–7.5 min, 40–20% B; 7.5–13 min, 20% B; 13–13.5 min, 20–60% B; 13.5–19 min, 60% B. Taxol levels were monitored at a wavelength of 227 nm and the retention time was 9 min 33 s. Two replicates for each cell culture sample were injected. Amino acid: 5 mL of cell culture was sampled and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, the supernatant was stored at −20 °C for HPLC quantification. The measuring method was according to Mou.19 Every sample was measured with two parallels.
000 rpm for 10 min, the supernatant was stored at −20 °C for HPLC quantification. The measuring method was according to Mou.19 Every sample was measured with two parallels.
3. Results
3.1 Verification of the viable biomass probe off-line
In plant cell culture, the amount of viable biomass is a crucial physiological parameter, which is highly correlated to cell growth, metabolism and the formation of the desired product. For quickly and effectively determining the cell concentration and metabolic state of Taxus chinensis var. mairei in a suspension culture, a radio frequency-impedance spectroscopy probe was used to estimate the viable biomass of Taxus cells. We compared the precision of off-line methodology measurements with determined capacitance through a viable biomass probe (Fig. 1), and established the relationship between the capacitance and real biomass concentration. The results showed that the capacitance increased with the rise of dry cell weight (DCW), even up to 23.6 g L−1. The correlation response model between the capacitance and the conventional off-line analytical DCW could be obtained (eqn (1))| DCW = 10.45 × capacitance + 40.51 | (1) |
 | ||
| Fig. 1 Relationship between capacitance and the Taxus chinensis var. mairei cell concentration (DCW). | ||
The linear relationship between the capacitance value and cell concentration illustrates that capacitance is an effective method to estimate reliably the cell concentration of Taxus chinensis var. mairei online in a stirred tank reactor.
3.2 Process conductivity feedback controlling strategies
During the Taxus cultivation process, the inorganic nutrient concentration is one of the important substances for cell growth, and was usually batch pulse added into the bioreactor during the subculture process.20 As well as the nutrient concentration distribution field in the scale up in the large reactor, the fluctuation of broth conductivity caused by the nutrient concentration and field always has a serious negative effect on the Taxus cells’ physiological state.21 Therefore, we carried out a detailed analysis on the physical and physiological states of the Taxol fermentation process, and investigated the changing of capacitance and conductivity with a viable biomass probe online, the exhaust CO2 concentration and calculated CER with gas-MS in-line, and the conventional off-line analytical DCW measured every 2 days as the time point. As shown in Fig. 2, with the cell growth, the viable cell capacitance increased from 50.12 to 99.52 pF cm−1 and the broth conductivity declined from 3.15 to 2.20 mS cm−1, which indicated the nutrients were consumed gradually. The respiratory parameter of CO2 evolution rate was also increased during cell growth. 100 mL of sterilized FM was added to the culture at 288 h, which lead to a rapid augmentation of conductivity. | ||
| Fig. 2 Profiles of Taxus cell cultivation at the early growth stage and a later feeding process in a stirred bank reactor. Symbols: CER (◆); capacitance (▲); conductivity (▼); DCW (■). | ||
The capacitance profiles showed that the cell growth rate might be limited during the early stages of fermentation with high conductivity, meanwhile, the cell growth rate was also restricted when the conductivity decreased lower than 2.4 mS cm−1 after 240 h of cultivation. Following the addition of a feed medium, the broth conductivity quickly increased to 3.46 mS cm−1, the Taxus cell growth rate was inhibited and the CER value did not change significantly. These results showed that the cell growth rate was closely correlated with the conductivity of the broth. Therefore, the effects of medium concentration on cell growth and productivity were investigated at various levels of broth conductivity, which was controlled at 2.4, 2.6, 2.8 and 3.0 mS cm−1 by adding FM.
3.3 Influence of MeJA concentration on cell growth and Taxol production
Methyl jasmonate (MeJA) has been widely used as an elicitor for high production of secondary metabolites in a plant cell suspension and it can effectively activate the initial genes in the corresponding pathway.22,23 To investigate the effects of MeJA concentration on Taxol biosynthesis based on DCW, 5 different gradients (1.0, 3.0, 6.0, 9.0 and 12.0 μmol g−1 DCW) were analyzed in 500 mL shake flasks, with a 200 mL working volume. The cells in the 5 groups were all simultaneously inoculated from the same bubble-column bioreactor. The production process was performed for 12 days with pulsed feeding with 1% (v/v) ME and 5% (v/v) B5 medium every 4 days, to ensure that the cells were not in a nutritionally deficient condition.The results of MeJA effects on biomass growth are summarized in Fig. 3A; cell growth was greatly inhibited when the concentration of MeJA increased from 1.0 μmol g−1 DCW to 12.0 μmol g−1 DCW, leading to the specific cell growth rate greatly decreasing from 0.08 d−1 to 0.02 d−1. However, the specific Taxol production rate increased with increased MeJA concentration, elevating to 6 μmol g−1 DCW, and the maximum specific Taxol production rate reached 1.57 mg g−1 DCW, which was 120% higher than that under a lower MeJA concentration of 1 μmol g−1 DCW (0.687 mg g−1 DCW). Meanwhile, the specific Taxol production rate was significantly inhibited with further increases in the MeJA concentration to 12 μmol g−1 DCW. Combining the results of the growth rate and the yield efficiency, a MeJA concentration of 6 μmol MeJA per active Taxus cell was selected as the optimum condition for further research.
3.4 Optimization of process control technology with dynamic physiological parameters
In present study, we investigated and optimized a controlled nutrient feeding and inducer MeJA concentration for Taxol production directed by the conductivity and capacitance detected with a viable biomass probe. 1.5 L of well cultured Taxus chinensis var. mairei cells cultured in a 10 L bubble column bioreactor were transferred into four parallel 5 L bioreactors with 1.5 L of initial nutrients, respectively. The broth conductivities of the 4 reactors were controlled at 2.40 ± 0.05, 2.60 ± 0.05, 2.80 ± 0.05 and 3.00 ± 0.05 mS cm−1, respectively, by continuous feeding FM online (Fig. 4A). A two-phase culture of Taxus chinensis var. mairei was carried out in the stirred bank reactors. First, Ag+ was added into the broth as the first elicitor (final concentration 50 μM) on the second day. At the 280 h cultivation point, 6 μmol MeJA per gram DCW was added for stimulating Taxol biosynthesis.The results showed that nutrient intensity consistent with conductivity has a significant effect on the specific cell growth rate (Table 1). When the broth conductivity increased from 2.40 ± 0.05 to 3.00 ± 0.05 mS cm−1, the logarithmic phase and specific growth rate were both inhibited. After a 50 hour lag phase, the specific cell growth rate reached as high as 0.14 per day under 2.40 and 2.60 mS cm−1 conditions, which was great higher than that under high conductivity conditions. The higher nutrient intensity was not suitable for cell growth, which might be caused by the high osmotic pressure under excessive extracellular nutrients.24,25 These could also be reflected from the physiological respiratory parameter of CER and viable cell concentration, which was in good agreement with the trend of capacitance. Meanwhile, the specific sucrose consumption rate was almost the same in all nutrient control conditions, which indicated that sucrose was not the rate-limiting factor in the 4 parallel investigations.
| Unit | Conductivity (mS cm−1) | ||||
|---|---|---|---|---|---|
| 2.40 ± 0.05 | 2.60 ± 0.05 | 2.80 ± 0.05 | 3.00 ± 0.05 | ||
| a SGR: specific growth rate.b AGR: average growth rate in logarithmic phase.c SSCR: specific sucrose consumption rate.d SPRm: the highest specific production rate. | |||||
| SGRa | d−1 | 0.14 | 0.15 | 0.10 | 0.07 |
| AGRb | g d−1 | 0.83 | 0.87 | 0.48 | 0.31 |
| SSCRc | mmol per g DCW per d | 0.11 | 0.11 | 0.14 | 0.13 |
| SPRmd | mg per g DCW per d | 651 | 850 | 419 | 387 |
| Yp/s | mg mmol−1 | 2.93 | 3.48 | 2.33 | 2.07 |
After MeJA solution addition at 280 h, the cell growth rate was inhibited by the intense stress of the elicitor and stopped proliferating immediately (Fig. 4B). However, the CER value still increased at a lower conductivity level. The higher Taxol biosynthesis rate was obtained at a lower conductivity level than that under higher conductivity levels. These findings suggest that intracellular metabolic activities were higher at lower conductivity, leading to a high biosynthesis rate of secondary production. After stagnating for 3 days, the biomass started a fast growth phase again with the broth conductivity controlled at lower conditions, and the growth rate was recovered and comparable to the former stage before MeJA addition. While the cell growth rates were greatly inhibited under the broth conductivity at 2.80 ± 0.05 and 3.00 ± 0.05 mS cm−1.
In order to understand the reasons about why the capacitance value was unchanged after adding MeJA and recovered after 3 days, cell viability was compared before and after MeJA addition by the TTC method. Cell viability declined about 30% after the addition of MeJA and rose to about 85% of the original level after 5 days (data not shown), which indicated that although Taxus had some capacity to adapt to a variation in its living circumstances, the elicitor of MeJA had a strong immediate impact on cell viability.
The variation in the Taxol yield during the cultivated period is shown in Fig. 5A. The results illustrated that hardly any difference in Taxol concentration appeared before MeJA addition at 280 h of cultivation. However, the accumulation rate of Taxol was distinguished greatly after the addition of MeJA. The highest specific Taxol production rate was obtained at the conductivity of 2.60 ± 0.05 mS cm−1 (Table 1), followed by 2.40 ± 0.05 mS cm−1 and 2.80 ± 0.05 mS cm−1 conditions. The final Taxol production rate demonstrated that 6.13 ± 0.08 mg Taxol per DCW was obtained when the broth conductivity was controlled at 2.60 ± 0.05 mS cm−1 (Fig. 5B). Meanwhile, when the broth conductivity was controlled at 3.00 ± 0.05 mS cm−1, the production rate (3.92 ± 0.06 mg g−1 DCW) was seriously restricted, which was nearly 36% lower than that under the low broth conductivity condition. Moreover, analysis of the Taxol yields to sucrose consumption (Yp/s) (Table 1) also showed that controlling broth conductivity at 2.60 ± 0.05 mS cm−1 could dramatically improve the conversion ratio of substrates to Taxol economically.
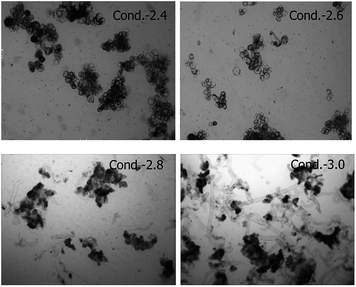 | ||
| Fig. 5 The morphology of Taxus chinensis var. mairei cells under different broth conductivity conditions at days 8 after MeJA addition. | ||
Fig. 5 showed the morphological feature of Taxus chinensis var. mairei under different broth conductivity conditions. Former research had revealed that cell aggregation is one unique characteristic of plant cell cultures because of the remaining connection via shared cell walls after division. Our results showed that the cells tend to aggregate with a decrease in conductivity, these might due to some nutrient diffusion limitations under the lowest broth conductivity condition. The large aggregate always has a negative effect on the oxygen and nutrient transfer into the cells, which must affect the cell biosynthesis.26 However, with an increase in broth conductivity, cell aggregation becomes smaller and some cells begin to elongate, with more and more elongated spindle formed cells appearing, especially when the broth conductivity reached 3.0 mS cm−1. Our results demonstrated that longer elongation of the single cell has a more negative effect on Taxol biosynthesis of Taxus chinensis var. mairei. Therefore, control of broth conductivity at 2.6 mS cm−1 could prevent well the large cell aggregation and control the extension of the single cell, which is important for cell growth, substrate transfer, and Taxol biosynthesis. The feedback control of the nutrient medium feeding directed by the online conductivity would be an effective method to avoid the negative effect of large conductivity fluctuation on cell growth and product biosynthesis of Taxus chinensis var. mairei.
3.5 Target extracellular amino acid analysis
Extracellular amino acid concentrations were detected to figure out the detailed variation in the cell. Two kinds of amino acids were found at high concentration during cell culture: phenylalanine (Phe) and leucine (Leu). As much research has reported, phenylalanine is an important precursor in the Taxol synthesis pathway and leucine can be utilized as a carbon and nitrogen resource for Taxol biosynthesis.27–29 As shown in Fig. 6, Phe and Lue greatly accumulated in the cell growth phase before 280 h and after adding the inducer MeJA, Phe concentrations greatly decreased, much more Phe was consumed and higher Taxol production rate was obtained (Fig. 6A). The Leu concentration was also decreased with Taxol biosynthesis after MeJA was added, but, there wasn’t any difference under the four conditions. Therefore, the variation of extracellular amino acids implied that controlling conductivity at 2.6 mS cm−1 is the best choice for precursor absorption and utilization. This information confirms that keeping Phe and Leu at a suitable concentration combined with a nutrient medium control with conductivity should be more effective for Taxol productivity.4. Discussion
The production of secondary plant metabolites using plant cell suspension cultures has become a much-used process. However, the valuable secondary products generated from plant cell cultures that reach the market greatly depend on the productivity and economics of the production process.21 Lots of research showed that cell lines with suitable genetic, biochemical, and physiological characteristics are obviously very important. The design of engineered Taxus cell lines with improved taxane production capacity is the challenge faced today in plant biotechnology.30 More recent developments have opened the possibility of cellular-based engineering of the plant Taxus cell.31,32 For example, Taxus culture transformation methods have recently been established. These advances are necessary for themes in genetic and metabolic engineering to commence. In this regard, Zhang et al. applied the direct pathway engineering via overexpression of the txs and dbat biosynthetic genes coding for taxadiene synthase and 10-deacetylbaccatin III-10 β-O-acetyltransferase, respectively, in transgenic T. chinensis. Results showed that the Taxol yield of the transgenic T. chinensis cells reached 35 μg per g DCW, which was about 70% higher than that of the non-transformed cells. Martínez-Márquez found a reliable protocol for the stable transformation using the Gateway-compatible Agrobacterium sp. binary vector system for fast reliable DNA cloning of non-embryogenic cells cultures of Taxus. These important achievements must encourage the implementation of metabolic engineering techniques in the production of taxanes in the future.Meanwhile, with the complete knowledge of the Taxol biosynthetic pathway, more and more research has focused on the potential for heterologous biosynthesis through an alternative production host, like E. coli, yeast or fungi.33 Lots of research has paid more attention to elucidating the steps required for Taxol biosynthesis, and also applied a hypothetical pathway to heterologously reconstitute the biosynthetic pathway.34 Production of Taxol intermediates at an early-stage pathway like isopentenyl diphosphate, dimethyl-allyl diphosphate and farnesyl diphosphate has been achieved in E. coli and yeast with genetic engineering strategies.21 Huang et al.33 exploited the conversion of GGPP to taxadiene catalyzed by taxadiene synthase in the committed step of Taxol biosynthesis, undoubtedly expediting progress toward elucidation of the molecular basis for catalysis of taxadiene formation and Taxol production. Jennewein et al. co-expressed Taxus cytochrome P450 reductase and cytochrome P450 oxygenases involved in Taxol biosynthesis in yeast to enhance product accumulation.34 But most heterologous expression of these genes always result in low expression levels.
Therefore, plant-derived Taxol biosynthesis remains a viable option for production purposes. Three species of T. canadensis, T. media and T. chinensis were mainly applied in research thus far.35 But paclitaxel productivity is significantly different among them; T. canadensis and T. media cell lines have a relatively higher paclitaxel productivity with less days in culture. Based on the research on the cell line of T. media and T. canadensis with optimized elicitation of methyl jasmonate, the highest production yield reached 110 mg L−1 and 117 mg L−1 during no more than a 2 week period, respectively.36,37 Meanwhile the T. chinensis cell line can achieve the highest paclitaxel production but over a longer period of days.38 The cell culture variability and low product yields are two limitations for plant cell culture technology.
For resolving these problems and effectively increasing paclitaxel production, strategies such as precursor and substrate feeding, media composition modification, two-phase partitioned organic solvent extraction, elicitor addition (including agents such as chitosan, silver ion and MeJA), and cellular dispersion (ultrasound) have been carried out.39 Choi et al. optimized a temperature-shift method for stimulating cell growth and paclitaxel production and the paclitaxel production reached 137.5 mg L−1 with an average productivity of 3.27 mg L−1 d−1 over 42 days.38 Bringi et al. reported the highest accumulation of 153 mg L−1 over a period of 42 days (equal to a productivity of 320 μg per g DCW per d) in T. chinensis cell cultures.35 Therefore, the Taxus cell lines have quite different Taxol productivity. In the present research on the cell line of Taxus chinensis var. mairei, the Taxol production reached 76.2 mg L−1 at 8 days after MeJA addition when the broth conductivity was controlled at 2.6 mS cm−1 constantly, and the highest specific production rate reached 850 μg per g DCW per d.
During the process control strategies, most culture optimization depends on precise maintenance of parameters such as pH, temperature, agitation, and dissolved oxygen content for scale up in larger reactors. The influence of different osmotic pressures generated by various sucrose concentrations, non-metabolic sugars, and non-sugar osmotic agents on Taxol production was investigated in suspension cell cultures of T. chinensis and the results showed that Taxol production could be enhanced with an initial osmotic pressure of 300 mOsm kg−1.40 By employing a semi-continuous T. canadensis suspension culture in conjunction with total cell recycling, Phisalaphong and Linden41 improved the Taxol specific production rate to 0.3 mg per g DCW per d during a 40 day continuous cultivation. Nevertheless, the Taxol production was always greatly reduced when scaled up to large bioreactors; the fluid mixing is another important aspect that must be considered.42–44
In large scale bioreactor, the shear force distribution, substrate concentration changes, oxygen transfer field and so on were proven to be the pivotal factors that affect successful scale up.45 Also, a long period of cultivation and difficult sampling process usually lead to contamination and are inconvenient for the judgement of the growth state. Therefore, online detection of the physiological parameters and nutrient concentration control were significantly important for scale up and process optimization of plant cell suspension culture.
In the present study, the effects of a broth conductivity controlling strategy directed by an online viable biomass monitoring sensor on the cell growth rate and Taxol productivity was investigated in Taxus chinensis var. mairei, suspension cultivation. A novel substrate feeding strategy based on online conductivity detected by a biomass monitor sensor was introduced to enhance the stability of fermentation, plant cell growth rate and the secondary production yield. Multiple parameters were recorded on-line or off-line, in particular capacitance, conductivity and CER. These measurements provide more detailed information in real time during cultivation and help formulate the theoretical basis for scaling up to an industrial process. Using an electrical method for the determination of the plant cell membrane was firstly introduced in 1981 where Zimmermann et al. used the charge-pulse technique to determine the membrane of giant algal cells of Valonia utricularis. This electrical method showed good agreement with the geometrical way.46 After that, this technology was gradually improved and applied extensively. Harker et al. used electrical impedance measurements to characterize changes in intracellular and extracellular resistance as well as changes in the condition of membranes during ripening of nectarines.47 November applied the biomass monitor to detect on-line viable biomass in wastewater, and found the advantage of a biologically more appropriate observation of the biomass compared to conventional dry weight measurements.48 Neves et al. monitored the concentration of Streptomyces clavuligerus in real time through a capacitance probe, which proved to be a valuable tool for real-time monitoring of biomass concentration in industrial-like cultivation.49 In 2013, Holland introduced radio frequency impedance spectroscopy into plant cell biomass measurements in-line and found that the biomass monitor has high resolution and was more accurate and rapid compared to the conventional method. It also can help to distinguish the cell form in different growth stage of plant cells.50
Although frequency impedance technology was widely applied in the biological field as mentioned above, in this study, we tried to emphasize the importance and feasibility of the conductivity feedback controlling strategy through the biomass monitor. It is quite suitable for the biological fermentation process especially for the plant cell suspension. Because plant cells have a larger size, this produces a high capacitance response and the composition of the plant cell medium is mainly electrolyte, which makes the conductivity measurement meaningful. We successfully controlled the conductivity of the culture based on the measurements with the biomass monitor. This approach ensured that the nutrients in the long period of culturing were stable and beneficial for cell growth and the production of Taxol. Meanwhile, the conductivity of the broth should also be controlled within defined limits, noting that levels higher than 2.8 mS cm−1 or lower than 2.4 mS cm−1 have negative effects on cell growth and Taxol production.
Providing an elicitor proved to be an effective strategy to induce and enhance the Taxol synthesis. Ag+ had a stimulating effect to improve the defense ability of Taxus cells, and helped to accommodate outside stimulation in the production phase.51 However, it should be borne in mind that an excess amount of elicitors can be toxic to cells and obviously affects their viability.2,22,23 It might be detrimental to the cells if an excessive amount of MeJA is added to the culture medium; it would result in inhibition of the regular growth of cells. Although we have no details about the mechanism on how MeJA affects the viability of cells, Patil45 has reported that MeJA suppresses growth by inhibiting progression of the cell cycle, an effect evident both at the culture and transcriptional level. Therefore, the elicitation strategy must be optimized for maintaining both cell activity and yield. In our study, although we obtained the best MeJA amount in the shake flask for final production, it inhibited the growth of cells immediately, and the effect will last for 2–3 days. Therefore, in the future, a suitable method will be developed, such as feeding MeJA solution continuously at low concentration, in order to stimulate the defense system of the cells to avoid inhibition of growth.
In conclusion, the biomass monitor probe is very useful for detecting plant cells during suspension cultivation in stirred tank reactors, since it avoids the need to take samples to measure dry cell weight and provides the real time viable cell concentration. The addition of MeJA (at 6 μmol g−1) is efficient for high production but is also harmful to the viability of cells; we intend in the near future to explore a better eliciting protocol. The conductivity of the culture plays a pivotal role in both cell growth and the yield of Taxol, both of which can be enhanced simultaneously when the conductivity is maintained at 2.6 mS cm−1.
Acknowledgements
This work was financially supported by a grant from the Major State Basic Research Development Program of China (863 Program), No. 2012AA021201, and 973 Program No. 2013CB733600. We would also like to warmly thank Guangdong Kelun Pharmaceutical Company for donating the industrial strain of Taxus chinensis var. mairei.References
- A. A. Kajani, S. Moghim and M. R. Mofid, Optimization of the basal medium for improving production and secretion of taxanes from suspension cell culture of Taxus baccata L., Daru, J. Fac. Pharm., Tehran Univ. Med. Sci., 2012, 20(1), 54 CrossRef CAS PubMed.
- R. M. Cusidó, J. Palazón, M. Bonfill, O. Expósito, E. Moyano and M. T. Piñol, Source of isopentenyl diphosphate for taxol and baccatin III biosynthesis in cell cultures of Taxus baccata, Biochem. Eng. J., 2007, 33(2), 159–167 CrossRef.
- S. Malik, R. M. Cusidó, M. H. Mirjalili, E. Moyano, J. Palazón and M. Bonfill, Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review, Process Biochem., 2011, 46(1), 23–34 CrossRef CAS.
- M. Onrubia, R. M. Cusido, K. Ramirez, L. Hernandez-Vazquez, E. Moyano and M. Bonfill, et al., Bioprocessing of plant in vitro systems for the mass production of pharmaceutically important metabolites: paclitaxel and its derivatives, Curr. Med. Chem., 2013, 20(7), 880–891 CAS.
- L. Li, X. Li, C. Fu, C. Zhao and L. Yu, Sustainable use of Taxus media cell cultures through minimal growth conservation and manipulation of genome methylation, Process Biochem., 2013, 48(3), 525–531 CrossRef CAS.
- S. A. Wilson and S. C. Roberts, Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules, Plant Biotechnol. J., 2012, 10(3), 249–268 CrossRef CAS PubMed.
- S. Garyali, A. Kumar and M. S. Reddy, Taxol production by an endophytic fungus, Fusarium redolens, isolated from Himalayan yew, J. Microbiol. Biotechnol., 2013, 23(10), 1372–1380 CrossRef CAS PubMed.
- S. K. Lenka, N. Boutaoui, B. Paulose, K. Vongpaseuth, J. Normanly and S. C. Roberts, et al., Identification and expression analysis of methyl jasmonate responsive ESTs in paclitaxel producing Taxus cuspidata suspension culture cells, BMC Genomics, 2012, 13, 148 CrossRef CAS PubMed.
- T. Wucherpfennig, J. Schilling, D. Sieblitz, M. Pump, K. Schütte and C. Wittmann, et al., Improved assessment of aggregate size in Taxus plant cell suspension cultures using laser diffraction, Eng. Life Sci., 2012, 12(6), 595–602 CrossRef CAS.
- J. Luo and G. He, Optimization of elicitors and precursors for paclitaxel production in cell suspension culture of Taxus chinensis in the presence of nutrient feeding, Process Biochem., 2004, 39(9), 1073–1079 CrossRef CAS.
- M. Onrubia, E. Moyano, M. Bonfill, R. M. Cusido, A. Goossens and J. Palazon, Coronatine, a more powerful elicitor for inducing taxane biosynthesis in Taxus media cell cultures than methyl jasmonate, J. Plant Physiol., 2013, 170(2), 211–219 CrossRef CAS PubMed.
- M. I. Georgiev, R. Eibl and J. J. Zhong, Hosting the plant cells in vitro: recent trends in bioreactors, Appl. Microbiol. Biotechnol., 2013, 97(9), 3787–3800 CrossRef CAS PubMed.
- H. Laukkanen, L. Rautiainen, E. Taulavuori and A. Hohtola, Changes in cellular structures and enzymatic activities during browning of Scots pine callus derived from mature buds, Tree Physiol., 2000, 20(7), 467–475 CrossRef CAS PubMed.
- R. A. Patil, M. E. Kolewe and S. C. Roberts, Cellular aggregation is a key parameter associated with long term variability in paclitaxel accumulation in Taxus suspension cultures, Plant Cell, Tissue Organ Cult., 2012, 112(3), 303–310 CrossRef PubMed.
- L. Li, Z. Wang, X. Chen, J. Chu, Y. Zhuang and S. Zhang, Optimization of polyhydroxyalkanoates fermentations with on-line capacitance measurement, Bioresour. Technol., 2014, 156, 216–221 CrossRef CAS PubMed.
- S. Zhang, B. Ye, J. Chu, Y. Zhuang and M. Guo, From multi-scale methodology to systems biology: to integrate strain improvement and fermentation optimization, J. Chem. Technol. Biotechnol., 2006, 81(5), 734–745 CrossRef CAS.
- M. A. Jermyn, Increasing the sensitivity of the anthrone method for carbohydrate, Anal. Biochem., 1975, 68(1), 332–335 CrossRef CAS PubMed.
- D. R. Duncan and J. M. Widholm, Measurements of viability suitable for plant tissue cultures, Methods Mol. Biol., 1990, 6(1064–3745), 29–37 CAS.
- M. Dehai, Determination of Amino Acids by Precolumn Derivatization with o-Phthaldialdehyde (OPA) and Reversed-Phase High Performance Liquid Chromatography, Chin. J. Chromatogr., 1997, 15(4), 319–321 Search PubMed.
- K. Zhao, W. Ping and D. Zhou, Recent advance and prospect on taxol production by endophytic fungus fermentation – a review, Weishengwu Xuebao, 2008, 48(3), 403–407 CAS.
- Y. Li, G. Zhang and B. A. Pfeifer, Current and emerging options for taxol production, Adv. Biochem. Eng./Biotechnol., 2015, 148, 405–425 CrossRef CAS PubMed.
- M. Bonfill, S. Bentebibel, E. Moyano, J. Palazón, R. Cusidó and R. Eibl, et al., Paclitaxel and baccatin III production induced by methyl jasmonate in free and immobilized cells of Taxus baccata, Biol. Plant., 2007, 51(4), 647–652 CrossRef CAS.
- M. Bonfill, O. Exposito, E. Moyano, R. M. Cusidó, J. Palazón and M. T. Pinol, Manipulation by culture mixing and elicitation of paclitaxel and baccatin III production in Taxus baccata suspension cultures, In Vitro Cell. Dev. Biol.: Plant, 2006, 42(5), 422–426 CrossRef CAS.
- S. M. Friedman, Salt sensitivity and cell permeability, J. Hypertens., 1991, 9(9), 789–798 CrossRef CAS PubMed.
- B. Kempf and E. Bremer, Uptake and synthesis of compatible solutes as microbial stress responses to high-osmolality environments, Arch. Microbiol., 1998, 170(5), 319–330 CrossRef CAS PubMed.
- G. M. Shang, J. C. Wu and Y. J. Yuan, Improved cell growth and Taxol production of suspension-cultured Taxus chinensis var. mairei in alternating and direct current magnetic fields, Biotechnol. Lett., 2004, 26(11), 875–878 CrossRef CAS PubMed.
- E. Bemani, F. Ghanati, A. Rezaei and M. Jamshidi, Effect of phenylalanine on Taxol production and antioxidant activity of extracts of suspension-cultured hazel (Corylus avellana L.) cells, J. Nat. Med., 2013, 67(3), 446–451 CrossRef CAS PubMed.
- A. G. Fett-Neto, S. J. Melanson, S. A. Nicholson, J. J. Pennington and F. Dicosmo, Improved taxol yield by aromatic carboxylic acid and amino acid feeding to cell cultures of Taxus cuspidata, Biotechnol. Bioeng., 1994, 44(8), 967–971 CrossRef CAS PubMed.
- V. Srinivasan, V. Ciddi, V. Bringi and M. L. Shuler, Metabolic inhibitors, elicitors, and precursors as tools for probing yield limitation in taxane production by Taxus chinensis cell cultures, Biotechnol. Prog., 1996, 12(4), 457–465 CrossRef CAS PubMed.
- O. Exposito, M. Bonfill, E. Moyano, M. Onrubia, M. H. Mirjalili and R. M. Cusido, et al., Biotechnological production of taxol and related taxoids: current state and prospects, Anti-Cancer Agents Med. Chem., 2009, 9(1), 109–121 CrossRef CAS PubMed.
- S. Kusari, S. Singh and C. Jayabaskaran, Rethinking production of Taxol (R) (paclitaxel) using endophyte biotechnology, Trends Biotechnol., 2014, 32(6), 304–311 CrossRef CAS PubMed.
- K. X. Huang, Q. L. Huang, M. R. Wildung, R. Croteau and A. I. Scott, Overproduction, in Escherichia coli, of soluble taxadiene synthase, a key enzyme in the Taxol biosynthetic pathway, Protein Expression Purif., 1998, 13(1), 90–96 CrossRef CAS PubMed.
- Q. Huang, C. A. Roessner, R. Croteau and A. I. Scott, Engineering Escherichia coli for the synthesis of taxadiene, a key intermediate in the biosynthesis of taxol, Bioorg. Med. Chem., 2001, 9(9), 2237–2242 CrossRef CAS PubMed.
- B. Engels, P. Dahm and S. Jennewein, Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production, Metab. Eng., 2008, 10(3–4), 201–206 CrossRef CAS PubMed.
- J. J. Zhong, Plant cell culture for production of paclitaxel andother taxanes, J. Biosci. Bioeng., 2002, 94(6), 591–599 CrossRef CAS PubMed.
- R. E. Ketchum, D. M. Gibson, R. B. Croteau and M. L. Shuler, The kinetics of taxoid accumulation in cell suspension cultures of Taxus following elicitation with methyl jasmonate, Biotechnol. Bioeng., 1999, 62(1), 97–105 CrossRef CAS PubMed.
- M. Jiang, G. Stephanopoulos and B. A. Pfeifer, Downstream reactions and engineering in the microbially reconstituted pathway for Taxol, Appl. Microbiol. Biotechnol., 2012, 94(4), 841–849 CrossRef CAS PubMed.
- H. Choi, S. Kim, J. Son, S. Hong, H. Lee and H. Lee, Enhancement of paclitaxel production by temperature shift in suspension culture of Taxus chinensis, Enzyme Microb. Technol., 2000, 27(8), 593–598 CrossRef CAS PubMed.
- C. Wang, J. Wu and X. Mei, Enhancement of Taxol production and excretion in Taxus chinensis cell culture by fungal elicitation and medium renewal, Appl. Microbiol. Biotechnol., 2001, 55(4), 404–410 CrossRef CAS PubMed.
- S. Kim, H. Choi, J. Kim, H. Lee and S. Hong, Effect of osmotic pressure on paclitaxel production in suspension cell cultures of Taxus chinensis, Enzyme Microb. Technol., 2001, 28(2–3), 202–209 CrossRef CAS PubMed.
- M. Phisalaphong and J. C. Linden, Kinetic studies of paclitaxel production by Taxus canadensis cultures in batch and semicontinuous with total cell recycle, Biotechnol. Prog., 1999, 15(6), 1072–1077 CrossRef CAS PubMed.
- R. Ketchum and D. Gibson, Paclitaxel production in suspension cell cultures of Taxus, Plant Cell, Tissue Organ Cult., 1996, 46(1), 9–16 CrossRef CAS.
- B. J. Kim, D. M. Gibson and M. L. Shuler, Effect of subculture and elicitation on instability of taxol production in Taxus sp. suspension cultures, Biotechnol. Prog., 2004, 20(6), 1666–1673 CrossRef CAS PubMed.
- M. C. Naill and S. C. Roberts, Flow Cytometric Identification of Paclitaxel-Accumulating Subpopulations, Biotechnol. Prog., 2005, 21(3), 978–983 CrossRef CAS PubMed.
- R. A. Patil, S. K. Lenka, J. Normanly, E. L. Walker and S. C. Roberts, Methyl jasmonate represses growth and affects cell cycle progression in cultured Taxus cells, Plant Cell Rep., 2014, 33(9), 1479–1492 CrossRef CAS PubMed.
- U. Zimmermann, R. Benz and H. Koch, A new electrical method for the determination of the cell membrane area in plant cells, Planta, 1981, 152(4), 352–355 CrossRef CAS PubMed.
- F. R. Harker and J. H. Maindonald, Ripening of Nectarine Fruit: Changes in the Cell Wall, Vacuole, and Membranes Detected Using Electrical Impedance Measurements, Plant Physiol., 1994, 106(1), 165–171 CAS.
- E. J. November and J. F. Van Impe, On-line viable biomass measurement and estimation of the specific growth rate of activated sludge from municipal wastewater treatment, Water Sci. Technol., 2001, 43(7), 97–104 CAS.
- A. A. Neves, D. A. Pereira, L. M. Vieira and J. C. Menezes, Real time monitoring biomass concentration in Streptomyces clavuligerus cultivations with industrial media using a capacitance probe, J. Biotechnol., 2001, 84(1), 45–52 CrossRef CAS PubMed.
- T. Holland, D. Blessing, S. Hellwig and M. Sack, The in-line measurement of plant cell biomass using radio frequency impedance spectroscopy as a component of process analytical technology, Biotechnol. J., 2013, 8(10), 1231–1240 CAS.
- H. K. Choi, J. H. Yun, S. I. Kim, J. S. Son, H. R. Kim and J. H. Kim, et al., Enhanced production of paclitaxel by semi-continuous batch process (SCBP) in suspension culture of Taxus chinensis, Enzyme Microb. Technol., 2002, 31(3), 368 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |



