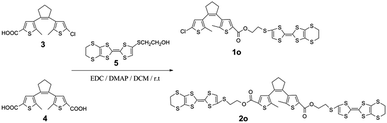
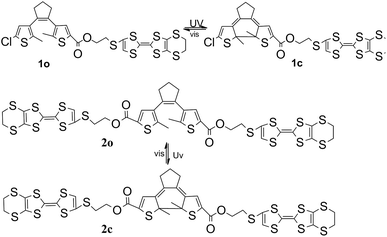
Novel multi-stimuli responsive molecules based on photochromic bithienylethenes containing the tetrathiafulvalene unit†
Jingyi Zhang,
Bingbing Sun,
Qiaochun Wang and
Lei Zou*
Key Laboratory for Advanced Materials and Institute of Fine Chemicals, East China University of Science & Technology, Shanghai, 200237, China. E-mail: zoulei@ecust.edu.cn; Fax: +86 21 64252758
First published on 2nd February 2016
Abstract
Two novel photochromic bisthienylethenes (BTEs) containing the tetrathiafulvalene (TTF) unit have been prepared. They are not only photochromic reversible, but TTF can achieve an electrochemically reversible conversion among TTF, TTF˙+/TTF22+ and TTF2+, accompanied with significant color changes. Explicit interactions between BTE and TTF units in the molecules can be observed.
Among various types of photochromic compounds, bisthienylethene derivatives (BTEs) are one of the most utilised and developed diarylethene structures, owing to their outstanding properties, such as thermally-irreversible photo-isomerization, excellent fatigue resistance and efficient photochromism performance.1 Upon alternating irradiation with UV and visible-light, the π conjugation of the BTE moiety shows striking changes due to C–C bond formation/disruption, thus affording a feasible approach to achieve photoswitchable electronic delocalization.2 In addition, such electrocyclic reactions can be induced not only upon photoirradiation but also by electrochemical reduction or oxidation.3 So far, research on photochromic diarylethene compounds has received much attention because of the distinguishable absorption spectra, changes in emission intensity, and other differences in physicochemical properties between the open and closed forms.4
Tetrathiafulvalene (TTF) chemistry has attracted significant interest since the discovery that the oxidized TTF˙+ form could be combined with suitable anions to give organic semi-conductors.5 As we know, the unique feature of TTF (and its derivatives) is that it can be sequentially oxidized to the radical cation TTF˙+ and dication TTF2+ through either chemical or electrochemical oxidations at easily accessible potentials.6 The 14 π-electron TTF unit is non-aromatic according to the Hückel rule, but its oxidation species, the radical cation TTF˙+ and dication TTF2+ are aromatic in the Hückel sense, which possesses aromaticity with one and two 6 π-electrons, respectively.7 And the electron absorption spectra of the TTF, TTF˙+, and TTF2+ are decisively different from one another.8 By making use of this feature, tetrathiafulvalene (TTF) derivatives have found applications in many areas of molecular electronics,9,10 ranging from organic field effect transistors (OFETs),11–13 optoelectronic materials,14 to molecular switches15–20 and photovoltaics,21,22 among others. To modulate TTF derivatives' unique electronic properties with a light input, a photochromic moiety is required. Thus, it is possible for us to design BTE derivatives containing TTF unit to achieve the desired reversible light response.
As mentioned above, upon alternating irradiation with UV and visible-light, the π conjugation of the BTE moiety shows striking changes. Herein, we have designed two novel BTE derivatives containing TTF units (Scheme 1, 1o and 2o) to explore whether the different isomeric forms of BTE moiety would affect the redox process of TTF unit. As a result, these two compounds exhibit good photochromic and electronic properties, and in the closed-ring isomer, BTE unit could act as the molecular wire to make the electrons of TTF disperse homogeneously throughout the whole molecule, so it become a little harder for TTF to lose electrons. There are explicit electronic interactions between BTE and TTF unit in the molecules.
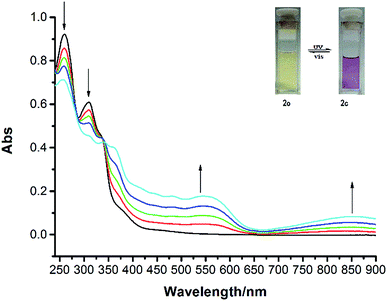
The photochromic properties of the target compounds were investigated in dichloromethane at room temperature. The photostationary state was reached upon UV irradiation at 254 nm, and the open-ring to closed-ring isomerization studies showed a fully reversible photochromic process of these two compounds (Scheme 2). Changes in the absorption spectra of compound 2o induced by photo irradiation at room temperature in CH2Cl2 (2.0 × 10−5 mol L−1) were shown in Fig. 1. For compound 2o, upon irradiation with 254 nm UV light, the photo-stationary state was attained after about 240 s and its conversion at photostationary state could reach 83%. The feature absorption of the BTE at 260 nm and 300 nm obviously decreased, while two new absorptions were observed at 550 nm and 850 nm. The absorption at λ = 550 nm was ascribed to the formation of the closed-ring isomer (2c), and the 850 nm absorption peak was attributed to the emergence of TTF22+,23,24 which could be an important evidence for the interactions between TTF and BTE unit. Accordingly, solution color changed from yellow to light purple gradually, using 517 nm light irradiation for some time, it turned to pale yellow again.
The absorption spectra of compound 1o irradiated by 254 nm UV light showed an analogous result, with the change of color from pale yellow to pink (see Fig. S1†). It took nearly 120 s to achieve the photo-stationary state and its conversion at photostationary state was 54%. In relative terms, the cyclization absorption of compound 2 overlapped with TTF to a broader absorption at 400 nm, and it had a more obvious absorption at 850 nm compared with compound 1. This may be ascribed to two TTF groups in compound 2, one as electron-donating group, which made it more conducive to the formation of TTF22+.
Practical application of photochromic technology requires photochromic molecules usually to be in polymeric matrices.25 Here, we prepared the PMMA films of compounds 1 and 2 to explore their optical properties in the solid state. The absorption spectral changes of solid films upon 254 nm photo-irradiation are shown in Fig. S2,† which were similar to that in CH2Cl2 solution. Moreover, we measured the cyclization quantum yields at 313 nm of the target compounds according to the literature.26–29 The cyclization quantum yields of compounds 1 and 2 are 0.06 and 0.07, respectively, which are relatively lower than the common diarylethenes (see Table S1†).
The oxidation potentials of 1o, 1c and 2o, 2c were determined by cyclic voltammetry. For comparison, the oxidation potentials of TTF-OH (compound 5, Scheme 1) under the same conditions were also included in Table 1. For TTF-OH, two reversible redox waves were observed at Eox1 = 0.54 V (TTF/TTF˙+) and Eox2 = 0.96 V (TTF˙+/TTF2+). Besides, both of compound 1 and 2 exhibited two redox couples which are typical for the TTF system. And reversible oxidation waves with significant differences were found between their open- and closed-ring isomers.
The cyclic voltammograms of 2o and 2c resemble their constituent unit—TTF, which were shown in Fig. 2. Two reversible redox waves for the open-ring isomer were observed at Eox1 = 0.52 V and Eox2 = 0.93 V (vs. Ag/AgCl), and an irreversible oxidation wave was observed at 1.40 V, which was attributed to the thiophene units oxidized to the cationic form.30 After solution 2o was exposed to UV light (254 nm) for 30 min (closed-ring isomer—2c formed), the oxidation wave at 1.40 V disappeared and the Eox1 and Eox2 anodically shifted around 30 mV and 40 mV (Table 1, ΔE), respectively.
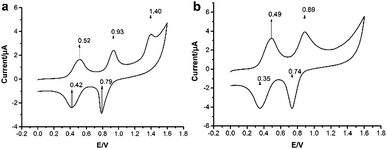 | ||
| Fig. 2 Cyclic voltammograms of compound 2 in CH2Cl2: 2o (a), 2c (b). 0.1 M tetrabutylammonium hexafluorophosphate as support electrolyte, with a scan rate of 50 mV s−1. | ||
Compound 2c compared with the isomer 2o, the decrease of Eox can be ascribed to the intramolecular electron transfer in BTE-TTF—the π-system of closed-ring BTE could act as the electron acceptor. Lower Eox of 2c indicated the reduction from cationic form to neutral form need less power, which demonstrated that at the closed-ring state of BTE, cationic forms of TTF could be formed more easily, and the electron mobility enhanced throughout the whole molecule. In addition, the disappearance of Eox = 1.40 V was because that before photo-irradiation, the thiophene units could lose electrons independently, while after illumination, the conjugated system extended to the whole thiophene rings which led to relative poor electron donors, in other words, BTE acted as molecular wire here, which made it become harder to lose electrons.
Similarly, 1c compared with 1o, Eox = 1.47 V disappeared, however, the Eox1 (0.56 V, vs. SCE) and Eox2 (1.00 V, vs. SCE) anodically shifted around 60 mV and 70 mV (Table 1, ΔE), respectively, which were higher than the reduced value of 2c (compared with 2o). This was because the Cl atom in 1c could also act as the electron acceptor (cyclic voltammograms see Fig. S3†). In summary, based on data above, we could draw a conclusion that there were explicit interactions between BTE and TTF units in the molecule.
Since electrochemical redox primarily related to the electric properties of TTF, here we mainly discuss the electronic change of TTF in compound 1 and 2, as well as the impact of BTE on the TTF unit.
As shown in Fig. 3, the characteristic absorptions of TTF-OH were observed at 300 nm and 325 nm. TTF-OH exhibited two reversible one-electron oxidation processes, corresponding to TTF/TTF˙+ and TTF˙+/TTF2+ redox reactions, respectively. TTF could be transformed into TTF˙+ and TTF22+ (π-dimer) by applying an oxidation potential at +0.65 V (vs. Ag/AgCl) and the formation of TTF2+ could be obtained by sequential oxidation potential at +1.1 V (vs. Ag/AgCl). Furthermore, TTF˙+, TTF22+ and TTF2+ could be reduced to the neutral TTF by applying a reduction potential at −1.1 V (vs. Ag/AgCl). It indicated that TTF could achieve reversible conversion at +0.65 V, +1.1 V, −1.1 V voltage.
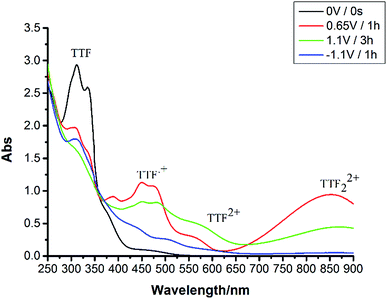 | ||
| Fig. 3 Absorption spectra of TTF-OH in CH2Cl2 (2.5 × 10−4 mol L−1) after applying different potentials. | ||
For compound 2o (Fig. 4a), while applying an oxidation potential at +0.65 V (vs. Ag/AgCl) for 1 h, the solution color changed from yellow to light purple. New obvious absorption bands at 450 nm, 480 nm and 850 nm appeared, and the absorption at 360 nm of neutral TTF decreased (due to the impact of BTE, the characteristic absorption of TTF red shifted). The absorption bands at 450 nm and 480 nm were attributed to TTF˙+ and 850 nm was ascribed to an intermolecular interaction production—π-dimer (TTF22+).31 Sequential application of an oxidation potential at +1.1 V (vs. Ag/AgCl) for another 5 h, with the change of color from light purple to dark purple, the absorption bands at 450 nm, 480 nm and 850 nm decreased with the emergence of absorption band at 620 nm which was attributed to TTF2+, due to TTF˙+ and TTF22+ were oxided. Then, by the application of a reduction potential at −1.1 V (vs. Ag/AgCl) for 1 h, there was almost no absorption at λ > 400 nm and the absorption at 360 nm appeared, indicating the reduction of TTF2+, TTF˙+/TTF22+ to the neutral TTF. Meanwhile, the solution color returned to yellow. This demonstrated that compound 2o could also achieve reversible conversion at +0.65 V, +1.1 V, −1.1 V voltage.
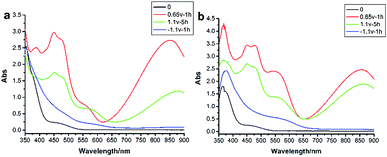 | ||
| Fig. 4 Absorption spectra of 2o (a) and 2c (b) in CH2Cl2 (2.5 × 10−4 mol L−1) after applying different potentials. | ||
Besides, applying an oxidation potential at +1.1 V, the absorption at 850 nm of 2c (Fig. 4b) decreased less than 2o, that was because, the BTE unit in 2c acted as the molecular wire to make the electrons of TTF disperse homogeneously throughout the whole molecule, which improved the stability of the molecule. This result was consistent with our CV experimental result as discussed above.
Similar spectral changes of the compound 1 were observed by applying same oxidative/reductive potentials (see Fig. S4†). It's worth mentioning that applying an oxidation potential at +1.1 V, the absorption at 850 nm of 1c showed almost no reduction. This was a joint result of the electron-withdrawing effect of Cl atom and the molecular wire role of closed-ring state of BTE.
Conclusions
In summary, we prepared two novel BTE derivatives containing TTF units. Their photochromic and electrochemical properties were investigated. These two compounds were both photochromic reversible and electrochemically reversible. They could be triggered by both light and electricity. By applying different potentials, distinct absorption bands of TTF, TTF˙+/TTF22+ and TTF2+ could be observed, accompanied with significant color change whether in the open- or closed-ring isomers. Above all, there were explicit interactions between BTE unit and TTF unit in these two compounds. Therefore, the combination of them formed a kind of efficient photoactive diarylethene derivatives with redox-modulation properties, which indicated they may have the potential applications in the photoelectric field.Acknowledgements
This work was financially supported by National Basic Research 973 Program (2013CB733700), National Natural Science Foundation of China (No. 21302056, 21372076, 21190033). Professor He Tian is also acknowledged for helpful discussion.Notes and references
- C. Li, W.-L. Gong, Z. Hu, M. P. Aldred, G.-F. Zhang, T. Chen, Z.-L. Huang and M.-Q. Zhu, RSC Adv., 2013, 3, 8967 RSC.
- G.-T. Xu, B. Li, J.-Y. Wang, D.-B. Zhang and Z.-N. Chen, Chem.–Eur. J., 2015, 21, 3318 CrossRef CAS PubMed.
- Y. Moriyama, K. Matsuda, N. Tanifuji, S. Irie and M. Irie, Org. Lett., 2005, 7, 3315 CrossRef CAS PubMed.
- J. C.-H. Chan, W. H. Lam and V. W.-W. Yam, J. Am. Chem. Soc., 2014, 136, 16994 CrossRef CAS PubMed.
- S. Adeel, M. E. Abdelhamid, A. Nafady, Q. Li, L. L. Martin and A. M. Bond, RSC Adv., 2015, 5, 18384 RSC.
- Y. Zhou, H. Wu, L. Qu, D. Zhang and D. Zhu, J. Phys. Chem. B, 2006, 110, 15676 CrossRef CAS PubMed.
- C.-G. Liu, Z.-M. Su, X.-H. Guan and S. Muhammad, J. Phys. Chem. C, 2011, 115, 23946 CAS.
- C.-C. Liu and X.-H. Guan, Phys. Chem. Chem. Phys., 2012, 14, 5297 RSC.
- J. L. Segura and N. Martín, Angew. Chem., Int. Ed., 2001, 40, 1372 CrossRef CAS.
- N. Martín, Chem. Commun., 2013, 49, 7025 RSC.
- N. Naraso, J.-I. Nishida, S. Ando, J. Yamaguchi, K. Itaka, H. Koinuma, H. Tada, S. Tokito and Y. Yamashita, J. Am. Chem. Soc., 2005, 127, 10142 CrossRef PubMed.
- M. Mas-Torrent and C. Rovira, J. Mater. Chem., 2006, 16, 433 RSC.
- M. Mas-Torrent and C. Rovira, Chem. Soc. Rev., 2008, 37, 827 RSC.
- F. Pop, A. Amacher, N. Avarvari, J. Ding, L. M. L. Daku, A. Hauser, M. Koch, J. Hauser, S.-X. Liu and S. Decurtins, Chem.–Eur. J., 2013, 19, 2504 CrossRef CAS PubMed.
- D. Canevet, M. Sallé, G. Zhang, D. Zhang and D. Zhu, Chem. Commun., 2009, 2245 RSC.
- K. Phoa, J. B. Neaton and V. Subramanian, Nano Lett., 2009, 9, 3225 CrossRef CAS PubMed.
- J. M. Spruell, A. Coskun, D. C. Friedman, R. S. Forgan, A. A. Sarjeant, A. Trabolsi, A. C. Fahrenbach, G. Barin, W. F. Paxton, S. K. Dey, M. A. Olson, D. Benítez, E. Tkatchouk, M. T. Colvin, R. Carmielli, S. T. Caldwell, G. M. Rosair, S. G. Hewage, F. Duclairoir, J. L. Seymour, A. M. Z. Slawin, W. A. Goddard III, M. R. Wasielewski, G. Cooke and J. F. Stoddart, Nat. Chem., 2010, 2, 870 CrossRef CAS PubMed.
- A. Coskun, J. M. Spruell, G. Barin, A. C. Fahrenbach, R. S. Forgan, M. T. Colvin, R. Carmieli, D. Benítez, E. Tkatchouk, D. C. Friedman, A. A. Sarjeant, M. R. Wasielewski, W. A. Goddard III and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 4538 CrossRef CAS PubMed.
- C. Simão, M. Mar-Torrent, J. Casado-Montenegro, F. Otón, J. Veciana and C. Rovira, J. Am. Chem. Soc., 2011, 133, 13256 CrossRef PubMed.
- A. C. Fahrenbach, S. C. Warren, J. T. Incorvati, A.-J. Avestro, J. C. Barnes, J. F. Stoddart and B. A. Grzybowski, Adv. Mater., 2013, 25, 331 CrossRef CAS PubMed.
- N. Martín, L. Sanchez, M. Á. Herranz, B. Illescas and D. M. Guldi, Acc. Chem. Res., 2007, 40, 1015 CrossRef PubMed.
- F. G. Brunetti, J. L. López, C. Atienza and N. Martín, J. Mater. Chem., 2012, 22, 4188 RSC.
- M. Iyoda, M. Hasegawa and Y. Miyake, Chem. Rev., 2004, 104, 5085 CrossRef CAS PubMed.
- Y. Feng, Q. Zhang, W. Tan, D. Zhang, Y. Tu, H. Ågren and H. Tian, Chem. Phys. Lett., 2008, 455, 256 CrossRef CAS.
- J. Jin and L. Zou, Chin. J. Chem., 2011, 29, 2445 CrossRef CAS.
- W. Li, X. Li, Y. Xie, Y. Wu, M. Li, X.-Y. Wu, W.-H. Zhu and H. Tian, Sci. Rep., 2015, 5, 9186 CrossRef CAS PubMed.
- W. Zhu, L. Song, Y. Yang and H. Tian, Chem.–Eur. J., 2012, 18, 13388 CrossRef CAS PubMed.
- X. Li, W. Li, H. Ågren, H. Tian and W. Zhu, Dyes Pigm., 2016, 125, 348 CrossRef CAS.
- Y. Wu, Y. Xie, Q. Zhang, H. Tian and W. Zhu, Angew. Chem., Int. Ed., 2014, 53, 2090 CrossRef CAS PubMed.
- E. C. Harvey, J. Areephong, A. A. Cafolla, C. Long, W. R. Browne, B. L. Feringa and M. T. Pryce, Organometallics, 2014, 33, 3309 CrossRef CAS.
- H. Spanggaard, J. Prehn, M. B. Nielsen, E. Levillain, M. Allain and J. Becher, J. Am. Chem. Soc., 2000, 122, 9486 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental methods and procedures, 1H NMR/13C NMR/MS of 1o and 2o, the photochromic parameters, UV-vis spectrums in PMMA films of compounds 1 and 2, and UV-vis, CV and electrochemical redox spectrums of compound 1. See DOI: 10.1039/c5ra26528b |
| This journal is © The Royal Society of Chemistry 2016 |