The cholesterol aided micelle to vesicle transition of a cationic gemini surfactant (14-4-14) in aqueous medium
Abstract
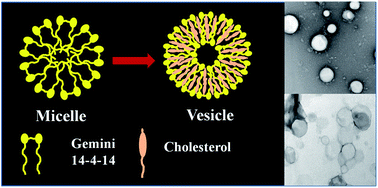
The cholesterol (Chol) aided micelle to vesicle transition of the cationic (14-4-14) gemini surfactant (GS) (tetramethylene-1,4-bis(dimethyltetradecylammonium bromide)) has been investigated employing spectrophotometry (visible and fluorescence), dynamic light scattering (DLS) and high resolution transmission electron microscopy (HRTEM) at different R (R = [Chol]/[GS]) values. Turbidity of the mixed solutions gradually increased with increasing R, and was highest at R = 1. There were hypsochromic spectral shifts with varied R, supporting transformation of micelles to vesicles, corroborated by DLS measurements. The vesicle sizes ranged between ∼160–240 nm. The steady state fluorescence anisotropy measurements suggested Chol induced anisotropy to the formed vesicles; they were structurally incongruent. The vesicle phase was found to be stable in the studied temperature range of 15–60 °C. The increased rotational relaxation time of the probe molecule C-153 (coumarin 153) supported enhanced rigidity of the local environment of the vesicles with an increasing proportion of Chol in the mixture.

 Please wait while we load your content...
Please wait while we load your content...