A large-scale on-chip droplet incubation chamber enables equal microbial culture time†
Abstract
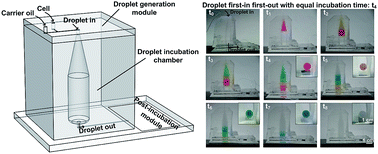
In many droplet-based screening applications, applying an equal incubation or reaction time to a large number of droplets is critical since differences in incubation time can lead to false positive or false negative hits during the selection process. Even though many droplet incubation platforms have been successfully utilized, having all droplets incubated for equal duration remains a formidable challenge. Here, we present an on-chip droplet incubation chamber that is capable of providing a constant droplet incubation time for a large number of droplets. This first-in first-out droplet incubation chamber essentially functions as a delay compartment to ensure that all droplets have equal incubation time inside the chamber before moving on to the post-incubation assay steps. The functionality of the developed chamber was tested by tracking color dye droplets flowing through the on-chip incubation chamber as well as comparing the growth of droplet-encapsulated cells of a filamentous fungus Fusarium verticillioides. The incubation time can be easily adjusted by changing the volume of the chamber or droplet collection speed. The chamber can also be easily integrated on-chip with other droplet microfluidics functional modules such as droplet generators, detectors, and sorters without any valve and tubing connection.

 Please wait while we load your content...
Please wait while we load your content...