Understanding the mechanism of replacement of citrate from the surface of gold nanoparticles by amino acids: a theoretical and experimental investigation and their biological application†
Monika Rania,
Lovika Moudgilb,
Baljinder Singhb,
Akshey Kaushalc,
Anu Mittald,
G. S. S. Sainib,
S. K. Tripathib,
Gurinder Singhe and
Aman Kaura*e
aCentre for Nanoscience and Nanotechnology, Panjab University, Chandigarh, 160014, India
bDepartment of Physics, Centre of Advanced Study in Physics, Panjab University, Chandigarh, 160014, India
cDepartment of Physics, IIT Delhi, 110016, India
dDepartment of Chemistry, Guru Nanak Dev University College, Chungh, Distt. Tarntaran, India
eDepartment of UIET, Panjab University SSG Regional Centre, Hoshiarpur, Punjab 146001, India. E-mail: amankaura1979@gmail.com; aman_kaura@yahoo.co.in; Fax: +91-1882-282221; Tel: +91-9501911977
First published on 1st February 2016
Abstract
The present study explores the physicochemical aspects needed for the appropriate in vitro synthesis and surface modification behavior of gold nanoparticles (AuNPs) in the presence of amino acids (AA). AAs replace citrate (Cit) from surface of citrate-coated AuNPs (Cit-AuNPs) depending upon their structural variations at different pH values. This behavior is further supported by molecular simulation studies, in which we have measured the effectiveness of different AAs in replacing the Cit from the gold (Au) surface. Within the limitations of the approach, the results indicate that cysteine (Cys) is most effective in replacing the Cit, arginine (Arg) is least effective, with glutamic acid (Glu) being intermediate. The UV-vis and FT-IR studies are employed to monitor the simultaneous synthesis and interactions of AA coated AuNPs over a range of pH values from 3.5 to 11. AA coated AuNPs are characterized by XRD and TEM studies. Cysteine coated AuNPs (Cys-AuNPs) show remarkable pH responsive behavior as compared to Glu and Arg coated AuNPs (Glu-AuNPs/Arg-AuNPs). Moreover, all AA coated AuNPs show little hemolysis with Arg-AuNPs having the least hemolytic effect. Thus, AA coated AuNPs are considered to be best vehicle for drug release and other biomedical applications.
Introduction
Amino acids are the most versatile biological materials that provide structural and mechanical support to cells, tissues and organs, composed of amine (–NH2) and carboxylic acid (–COOH) functional groups along with a side-chain specific to each AA. The mechanism of metabolism and functioning of AAs in the body is largely based upon their polarity,1 the dielectric behavior of the surrounding environment2,3 and the pKa values of the functionalities.4 Because of their biological significance, AAs are used in nutritional supplements,5–7 fertilizers,8,9 food technology,10,11 and industrial uses that include the production of drugs,12–14 and biodegradable15 and chiral catalysts.16,17Likewise, metal nanoparticles (NPs) have increasingly found practical applications in research and therapeutics18 due to their small particle size coupled with their unique chemical and physical properties. For such applications, stable AuNPs in solution are synthesized by coating their surfaces with different ligands.19–22 This coating also provides a method to control the activity of AuNPs by possible surface functionalization. AuNPs of different shapes are primarily stabilized by citrate anions in the synthesis process.23 However, citrate coated AuNPs are toxic in nature.24 In order to reduce the toxicity, an alternative approach has been developed; by which bio-functionalization of AuNPs can be achieved via the displacement of citrate from citrate-capped NPs. Sethi et al. reported that they are able to form linear, chain-like, nano-assemblies after partially replacing citrate by the AA arginine on the surface of AuNPs.25,26 Zhang et al. have studied the influence of salt concentration, pH and oligomer length on DNA absorption/desorption onto Cit-AuNPs.27 In addition, AA are used to increase the compatibility of metal nanoparticles to the living systems. Sharma et al. have reported bioconjugated Cys as a capping agent for the synthesis of AuNPs.28 The prepared AuNPs may find potential applications in various fields of biological and chemical sciences as biologically conjugated materials. Annadhasan et al. have synthesized silver and gold nanoparticles using tyrosine under natural sunlight irradiation that are very promising for colorimetric detection of Hg2+, Pb2+, and Mn2+ in aqueous medium.29 Liu et al. have reported the synthesis of glutamic acid stabilized gold nanoparticles which are assembled into one-, two-, and three-dimensional superstructures.30 Glutamic acid bound AuNPs provide implications to the exploitation for biological detection and biosensors. Grosan et al. have designed and fabricated modified electrodes by linking leucine capped AuNPs to 1,3-propanedithiol self-assembled monolayer on gold substrate.31
Thus, it has become necessary to further understand the mechanism behind driving adsorption of bio molecules on the surface of AuNPs. Despite recent advances in various experimental techniques,32,33 it remains challenging to study these materials, whilst data at this can in principle be analyzed by theoretical techniques. The phenomena at the atomistic level are quite complex, however significant progress has been made which allows us to use computational techniques to further understand the adsorption of biomolecules on the surface of Au. To our knowledge, there have been no studies based on simulations methods that probe the displacement of Cit on gold surface by AA under aqueous conditions. Computational techniques used to unravel the interactions at the atomistic level can support the experimental research in an efficient manner.34
Further, no study is found where Cys, Glu and Arg coated nanomaterials are employed to transport drugs through the bloodstream to target site-specific therapeutic applications. Thus, in order to explore the biomedical applications of AA coated AuNPs, the experimental aspects of citrate stabilized AuNPs and sequential surface modification with all the three AA under a pH range from 3.5, 5, 7, 9 and 11 have been evaluated. Their optical and microscopic behavior in relation to stability and aggregation has been explained with respect to the change in pH. All the three selected AA acids show aqueous phase solubility, that pave the way of its potential use in aqueous phase or aqueous soluble biodegradable materials. Further, the experimental studies are reproduced with atomistic simulation studies to measure the effectiveness of different AA in replacing the Cit from the surface of gold slab in the neutral aqueous environment. The biomaterials thus synthesized and characterized have been further subjected to hemolytic analysis, which is considered to be more relevant for their possible transportation in systematic circulation.
Material and methods
Hydrogen tetrachloroaurate(III) trihydrate (HAuCl4·3H2O), trisodium citrate, sodium borohydride, L-cysteine, L-arginine and L-glutamic acid are purchased from Sigma Aldrich, India. The pH of the Au colloidal solution and of AA solutions is adjusted separately using 1 N HCl and 1 N NaOH. The glassware is washed with aqua regia (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 = 3
HNO3 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)) and rinsed well with doubly distilled water prior to use.
1 (v/v)) and rinsed well with doubly distilled water prior to use.
Synthesis of AuNPs
Colloidal solution of AuNPs of concentration 0.5 mM is prepared. In a typical experiment, 25 ml of the 0.5 mM HAuCl4 is mixed with 5 mM trisodium citrate in doubly distilled water. This solution is then vigorously stirred for 35 min. To this solution, 0.6 ml of freshly prepared NaBH4 having concentration 0.002 M is added drop wise with constant stirring for about 1 min. This prepared solution is then kept undisturbed overnight. This prepared solution is characterized using UV-vis spectrometer showing Surface Plasmon Band (SPB) at approximately 520 nm (ref. 35).Preparation of amino acid (AA) solutions and AA coated AuNPs
All the three AA (Cys, Glu and Arg) of concentration 1 mM are prepared separately in doubly distilled water. 1 mM of each AA is taken in five separate 15 ml vials. The pH of each vial is adjusted as 3.5, 5, 7, 9 and 11 respectively. Similarly, the Au sol is also taken in five vials and pH of each is adjusted as 3.5, 5, 7, 9 and 11 respectively. The Au colloidal solution and the AA with adjusted pH separately are then mixed in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ratio respectively. The time effect, SPB, shift in intensity is measured with UV-vis spectrometer at a resolution of 1 nm and within the 200–900 nm wavelength range. Similarly the samples for TEM, FT-IR and XRD of the same solutions are prepared by centrifuging each solution at 10
1 (v/v) ratio respectively. The time effect, SPB, shift in intensity is measured with UV-vis spectrometer at a resolution of 1 nm and within the 200–900 nm wavelength range. Similarly the samples for TEM, FT-IR and XRD of the same solutions are prepared by centrifuging each solution at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and after washing at 10
000 rpm for 30 min and after washing at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. Two washings are carried out in each solution.
000 rpm for 10 min. Two washings are carried out in each solution.
Spectroscopic and microscopic analysis
UV-vis spectroscopy measurements are carried out on Hitachi U-3900 spectrometer operated at a resolution of 1 nm. Spectra are collected over a range of 200–900 nm. The FT-IR spectra are recorded using a Perkin Elmer Spectrum 400 spectrometer with 1 cm−1 resolution with KBr pellets. The XRD studies are carried out with ‘X’-Pert PRO, PANalytical of all AA capped Au nanoparticle samples at variable pH conditions. The samples are taken on a glass slide and the diffractogram are recorded using nickel-filtered CuKα radiation at a scanning rate of 0.6 deg 2(theta) per min. The TEM analysis are conducted by placing a drop of working solution on carbon coated copper grid and followed by solvent evaporation at room temperature.pH measurements
An Accumet digital pH meter 910, fitted with a glass electrode is used for pH measurements of all AA and AuNPs solutions separately.Hemolysis
Hemolytic assay is performed to evaluate the response of Cys, Glu and Arg coated AuNPs on blood group AB of Red Blood Cells (RBCs) from a healthy human donor. Briefly, 5% suspension of RBCs is used for this purpose after three washings along with two concentrations (i.e., 25 and 50 μg ml−1) of each NPs sample. 1 ml packed cell volume (i.e., hematocrit) is suspended in 20 ml of 0.01 M phosphate buffered saline (PBS). The positive control is RBCs in water, and it is prepared by spinning 4 ml of 5% RBCs suspension in PBS. PBS as supernatant is discarded, and pellet is resuspended in 4 ml of water. The negative control is PBS. All the readings are taken at 540 nm, i.e. absorption maxima of hemoglobin.Ethical statements
In the present study, 5 ml of human blood is used in a controlled manner in our laboratory. All the experiments are performed for best practice according to the Panjab University guidelines. Further, consent of the donor has been taken before performing the experiment. We did not conduct any experimentation with animal subjects.Computational methods
Classical molecular dynamics (MD) simulations of AA adsorbed on the Cit capped Au(111) surface in explicit water has been done using simulation package GROMACS, Groningen Machine for Chemical Simulations program (version 4.6.5)36 with the SPC/E model for water.37,38 The simulations are performed with the GOLP force field.39,40 Simulations are done in parallelepiped boxes with periodic boundary conditions. A gold slab of 6.06 nm × 5.57 nm (thickness of five atomic layers) is used. Citrate surface coverage (1 citrate nm−2) is used on the Au(111) surface. The three AA (Cys, Glu and Arg) are independently placed on the Cit covered Au slab. The system is subjected to equilibration in which first of all water molecules are equilibrated for 2 ns (all other molecules are held fixed). This is then followed by geometry optimization of all the atoms. The system is then subjected to simulated annealing, with following schedule: a 2 ns simulation of all atoms at 310 K, followed by MD run for 2 ns at 610 K, at 310 K for 2 ns, at 610 K for 2 ns and finally at 310 K for 2 ns. The temperature of the system is maintained by coupling the system to a Nose–Hoover thermostat bath. The total time period for equilibration is 12 ns. During equilibration, Cit–gold desorption and interactions between different AA are prevented by restraining the carbon atom of the Cit and fixing the Cα carbon atoms of the AA. We have selected the box size such that AA, Cit and Au with their periodic images are separated by at least 12 Å and the entities of the system are not able to interact with its periodic images via van der Waals potentials. Simulations are performed in physiological ion concentration (150 mM NaCl). All simulations are conducted in the canonical NVT ensemble with a time step of 1 fs. After annealing simulations are done for 15 ns with all restraints removed.Results and discussion
Time effect
UV-vis spectra of as made colloidal Cit-AuNPs at pH range 3.5, 5, 7, 9 and 11 (ESI, Fig. SF-1†) over a time span of 60 minutes are used for comparison in each experiment. Colloidal Cit-AuNPs sol exhibits a distinctive SPB at 520 nm at various pH values except pH 3.5. The excitation peak is one of the size dependent properties of AuNPs. Any change to the NPs dielectric environment such as molecules binding to the surface of AuNPs or NPs aggregation shift the spectral weight to longer wavelengths.41 Likewise, the aqueous solutions of AA with the same pH range are made and mixed with Cit-AuNPs sol and the changes in UV-vis spectra are measured. Fig. 1, ESI, Fig. SF-2 and SF-3† clearly depict the changes in absorbance and SPB of AA-AuNPs at different pH values with time. The photographs of the mixed solutions of Cit-AuNPs and AA at 0 min and after 60 minutes of the reaction are also shown in ESI, Fig. SF-4.† There is no color change in the presence of Glu and Arg while in the case of Cys, there is instant color change at all pH values, which is further supported from UV-vis studies and is discussed later in this section. | ||
| Fig. 1 UV-vis scans of a reaction of Cys with citrate capped AuNPs under the effect of reaction time (in minutes) at different pH values (a) pH 5 (b) pH 7 (c) pH 9 and (d) pH 11. | ||
In the case of Cys-AuNPs, at pH 5 and pH 7, there is a broad band at ∼570 nm (Fig. 1a and b). With the passage of time, the absorbance decreases and after 2 h the solution is clear with aggregates being settled down. However, at pH 9 (Fig. 1c), there is distinct band at 520 nm with a shoulder at ∼630 nm. At pH 11 (Fig. 1d), there is appearance of two bands at 520 nm and 650 nm. In both the cases, appearance of bands at longer wavelength is accompanied by color change of the solution from red to purple to blue. This evolution at all pH values reflects the decrease of free AuNPs and formation of Cys mediated nanoparticle assembly in the solution.
In Glu-AuNPs (ESI, Fig. SF-2†) and Arg-AuNPs (ESI, Fig. SF-3†), SPB remains same ∼520 nm at all pH values except pH 3.5. Moreover, at a particular pH, there is no change in absorption with time, which indicates the stability of Glu-AuNPs and Arg-AuNPs. However, at pH 3.5, the solution shows aggregation with color change from red to purple and then blue.
The above mentioned analysis determines the tendency of different AA to bind to the AuNPs and to induce aggregation. The stability of colloidal particle is best explained on the basis of DLVO (Derjaguin–Landau–Verwey–Overbeek) theory in terms of flocculation and coagulation.42 The forces which are responsible for the interaction among colloidal particles are attractive van der Waals forces (VA) and repulsive Coulomb forces (VR). Obviously, the dispersed colloid is stable when VR ≫ VA whereas VR ≪ VA leads to aggregation. Therefore, in the colloidal solution several factors such as particle size, surface potentials and electric double layer influence the stability of NPs and their aggregation. NPs are stable in solution due to electrostatic repulsion of their charged surface. Lack of sufficient charge or stabilizing agent will cause the particles to aggregate or precipitate. In our case, Cit ions are adsorbed onto the surface of as prepared AuNPs, creating a negative surface charge that stabilizes the particles. Before the addition of AA molecules, the Au particles are colloidal stable since energy barrier is high enough to prevent aggregation. Addition of AA to the Cit-AuNPs disrupts the Cit layer to different extent depending upon the pH of the media. Moreover, the variation in the aggregation kinetics among these selected small molecules can be attributed to the differences in their functional groups ability to interact with the AuNPs. Au sol is stabilized by anionic Cit molecules, which are adsorbed on the AuNPs surface at all pH values except at 3.5, where Cit molecules itself undergo protonation. Further on addition of Cys at pH 3.5, 5, 7, 9, 11, the thiol group undergoes strong and thermodynamically favorable covalent bond formation with Au surface. Due to strong covalent bond (soft–soft interaction), –SH group will replace citrate group, thus adsorbed on the surface of AuNPs and NH2 (pH 9 and pH 11), NH3+ (at lower pH) and COO− groups will remain on the surface. The –SH group, which interacts strongly through strong covalent bonding, is independent of pH. However, plasmon coupling occurs due to the formation of inter nanoparticle bridges due to H-bonding between NH3+ (at pH 5 and 7) and NH2 (at pH 9 and 11) present on one Cys-AuNPs surface and COO− of other Cys-AuNPs surface. In addition to this, electrostatic interactions between NH3+ and COO− are also responsible for such aggregation at pH 5 and 7. Further at pH 3.5, more aggregation occurs due to combined effect of internanoparticle bridges, electrostatic interactions and protonation of all COO− groups of citrate molecule as pKa values of citrate are 3.14, 4.77, 6.39.43 TEM Studies at pH 11 of Cit-AuNPs (Fig. 2a), Cys-AuNPs at 0 minutes (Fig. 2b) and 60 minutes (Fig. 2c) after the reaction also support the results. At 0 minutes i.e. at the start of the reaction, particle size increases up to 8 nm along with the indication of formation of inter nanoparticles bridges as compared to the monodispersed Cit-AuNPs of size up to 4 nm. After 60 minutes, there is clear indication of formation of the inter nanoparticle bridges among the Cys-AuNPs along with few monodisperse particles.
 | ||
| Fig. 2 TEM images and particle size distribution of (a) citrate capped gold nanoparticles (b) Cys-AuNPs at 0 minutes (c) Cys-AuNPs at 60 minutes. | ||
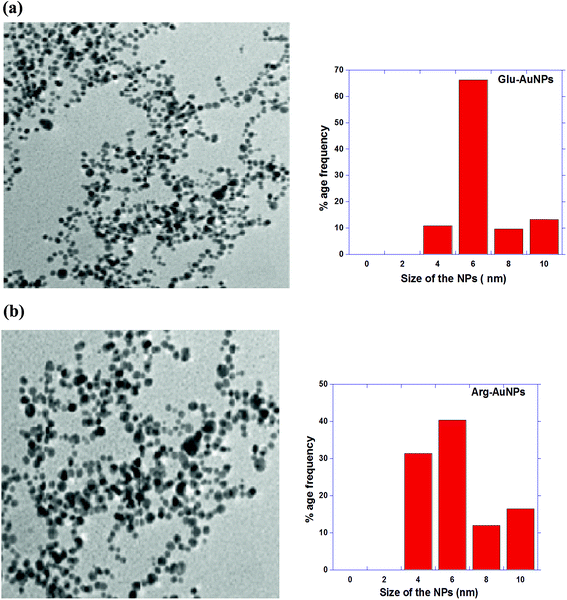
In the case of Glu-AuNPs, no aggregation is seen at various pH values from 5 to 11 (ESI, Fig. SF-2†). This may be attributed to two COO− groups of Glu, which stabilize the AuNPs with similar interactions as COO− groups of Cit molecule adsorbed on AuNPs surface. These results are further supported from computational studies in the next section. This effect is continued till all COO− groups of Glu and adsorbed citrate undergo protonation which occurs below pH less than 4. At higher pH values, the weaker covalent bonds of –NH2 along with the electrostatic interactions of COO− of Glu and citrate molecules, remain adsorbed on AuNPs surface and one or two carboxyl groups face outwards to stabilize the AuNPs. Similarly, in the case of Arg-AuNPs (ESI, Fig. SF-3†), one of the amine acts as primary amine can interact with AuNPs again through weak covalent bonds along with COO− of Arg and Cit molecules. The Arg molecule has pKa1, pKa2 and pKa3 values 2.17, 9.04 and 12.48 respectively. With a pKa3 of 12.48, the guanidinium group is positively charged in neutral, acidic, and even most basic environments, and thus imparts basic chemical properties to Arg. Because of the conjugation between the double bond and the nitrogen lone pairs in guanidinium group, the positive charge is delocalized and does not show strong electrostatic interactions with neighboring COO− groups, which leads to the stabilization of Arg-AuNPs. Glu and Arg coated AuNPs also show monodispersity with average particle size of 6 nm (Fig. 3a and b). These results also are supported by computational studies.
Concentration effect
The concentration effect of all AA is studied on Cit-AuNPs over the range (0.01–0.05 mM) at pH 11. The concentration at which aggregation takes place is determined by carrying out a titration, in which [Cys] = 1 mM is added to Cit-AuNPs to get aggregation point. There is clear aggregation point at pH 11 (Fig. 4a), which occurs at [Cys] = 0.03 mM. In contrast, the UV-vis data of both Glu-AuNPs and Arg-AuNPs shows no apparent change in both SPB and in absorption intensity. No inflection point (Fig. 4b) conferring no aggregation of Glu-AuNPs and Arg-AuNPs is obtained suggesting the stability of respective AA-AuNPs. | ||
| Fig. 4 Concentration titrations to determine the aggregation point (a) Cys-AuNPs at pH = 5 and 11 (b) Glu-AuNPs and Arg-AuNPs at pH 11. | ||
Interaction and mode of attachment
The interaction of AA on the surface of AuNPs is further confirmed by detailed FTIR characterization of pure AA, AA and AA-AuNPs at various pH values (ESI, Fig. SF-5–SF-8†). In the pure Cys (ESI Fig. SF-5a†), the band at 1593 cm−1 and 1421 cm−1 corresponds to symmetric and asymmetric stretching of COO− and a very broad band at 3177–2636 cm−1 is due to N–H stretch. –SH group shows a weak stretching band at 2549 cm−1. The results are in good agreement with typical AA.44 But changing the dielectric environment of Cys molecule to slightly acidic, pH = 5 (ESI Fig. SF-5b†), conditions, the bands at 3177 and 2636 cm−1 merge and shift to 3399 cm−1, bands S–H stretch appear at 2600 cm−1, bands at 1593 cm−1 and 1421 cm−1 shifts to 1642 cm−1 and 1405 cm−1, band at 1061 cm−1 due to C–N stretch shift to 1035 cm−1 and band at 865 cm−1 due to N–H out of plane bending shift to 795 cm−1. IR spectra of Cys-AuNPs at pH = 5 (ESI, Fig. SF-5c†) shifts the stretching band at 3399 cm−1 due to N–H stretch (Cys pH = 5) to 3411 cm−1. Bands at 1642 cm−1 and 1405 cm−1 due to COO− shift to 1644 cm−1 and 1422 cm−1. Bands at 1035 cm−1 due to C–N stretching shift to 1077 cm−1 and band at 795 cm−1 due to N–H out of plane bending disappear. S–H stretching at 2600 cm−1 disappears indicating the covalent interaction of Au–S. Under basic conditions at pH 11 (ESI, Fig. SF-6a†), –SH stretching band disappear in Cys at pH 11 due to acidic nature of –SH bond. The bands at 3364 cm−1 and 3193 cm−1 (due to N–H stretch), 1077–1020 cm−1 (due to N–H bending) shift to 3366 cm−1, 3200 cm−1 and 1100 cm−1. Moreover, band at 864 cm−1 due to N–H out of plane bending disappear. Bands at 1628 cm−1 and 1412 cm−1 (ESI, Fig. SF-6b,† Cys-AuNPs at pH = 11) due to COO− stretching shift to 1644 cm−1 and 1433 cm−1. Such shifts in bands support the adsorption of Cys on AuNPs under basic conditions. It is also proposed that S−, being soft base also plays a major role in NP formation, which is supported from shifts in the N–H and COO− peaks.FTIR spectra of pure Arg (ESI, Fig. SF-7a†) shows a broad merged bands at 3500 cm−1, 3346 cm−1, 3288 cm−1, 3106 cm−1, 2952 cm−1, 2868 cm−1 due to N–H stretching, bands at 1685 cm−1, 1645 cm−1, 1605 cm−1, 1563 cm−1, and 1471 cm−1, 1421 cm−1 and 1329 cm−1 due to stretching of C–O bond in COO− and N–H bending bands at 1009 cm−1 due to C–N stretching, band at 850 cm−1 due to N–H bending out of plane. At pH = 5 (ESI, Fig. SF-7b†), stretching frequencies of Arg shows a shift when compared with FTIR spectra of pure Arg. Broad band due to N–H stretching merge and shift to 3392 cm−1, bands due to C–O stretching and N–H bending also merge to 1643 cm−1 and 1426 cm−1, band due to C–N stretching shift to 1065 cm−1 and band due to N–H out of plane bending shifts to 816 cm−1. FTIR spectra of Arg-AuNPs at pH 5 (ESI Fig. SF-7c†) again show a shift in the signals. Bands now shift to 3400 cm−1, 1644 cm−1, 1433 cm−1, 1077 cm−1 and band due to N–H out of plane bending at 816 cm−1 disappears. All these shifts indicate involvement of Arg in Au-NPs. At pH = 11 (ESI, Fig. SF-8a†), stretching frequencies again shows a shift when compared with FTIR spectra of pure Arg. Broad band due to N–H stretching merge and shift to 3308 cm−1, 3190 cm−1, 2926 cm−1, bands due to C–O stretching and N–H in plane bending merge and shift to 1635 cm−1 and 1424 cm−1. C–N stretching frequencies shift to 1097 cm−1 and 1019 cm−1. There is no band for out of plane N–H bending. FTIR spectra of Arg-AuNPs (ESI, Fig. SF-8b†), again shows a shift in stretching frequencies indicating the involvement of Arg in Au-NPs formation. Bands shifts to 3312 cm−1, 3195 cm−1, 1640 cm−1, 1417 cm−1 and 1089 cm−1.
XRD patterns are shown in ESI, Fig. SF-9,† and all diffraction peaks can be indexed to Au fcc geometry with predominant growth at (111) crystal planes. The peaks at 2Φ = 38.3, 44.6, 64.7, 77.5 are indexed as (111) (200) and (311) planes for AuNPs. The results agree well with reported standards (JCPDS file no. 04-0784).
Atomistic molecular dynamics (MD) simulations of AA on citrate covered Au
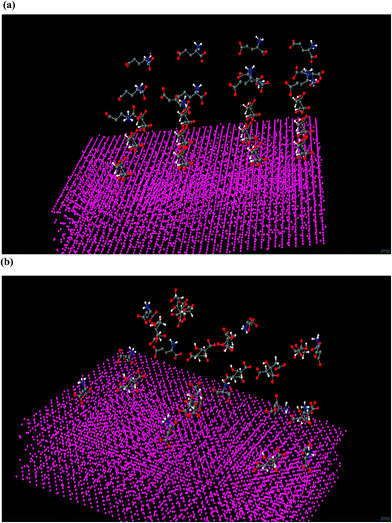
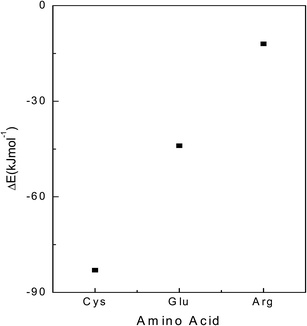
Twelve Cit anions are placed on the surface of gold slab at a distance 3–5 Å from the Au atoms. Equivalent numbers of AA are placed at a distance of 10 Å from the gold atoms. The system is first subjected to equilibration as per the procedure mentioned in the section computational methods. The system is then subjected to unrestrained MD simulation for 15 ns. In this simulation, interaction between different Cit anions is neglected as each one of them is placed at a distance 1 nm from each other. Similarly, the interaction between different AA is not considered as they are also placed at a distance of 1 nm from each other. Three different types of AA are considered Cys, Arg and Glu. Twelve AA of different types are placed independently on the citrate capped gold surface. We have not varied the pH, and have considered the available structure of AA at a neutral pH. For Cys, three different types of configurations are considered: one with –SH group and (–COO−) groups oriented towards the Au surface, another with –SH and –NH3+ groups oriented towards the slab and a third with only –SH group towards the Au. In case of Glu, we have considered three different types of orientations: one in which only one (–COO−) group is oriented towards gold, the one in which both the (–COO−) groups are oriented towards the gold slab and the final confirmation in which –NH3+ is oriented towards the gold slab. In case of Arg, we have considered its orientation such that one where the AA are adsorbed on the gold surface with their (–COO−) group and the other with their guanidinium group toward the gold slab. For each amino acid surface orientation, 5 identical configurations but with different sets of randomly Maxwell distributed atomic velocities are randomly generated and they are subjected to unrestrained MD simulations for 15 ns. In case of Cit-capped with Cys amino acid studies, MD results indicate its adsorption via –SH group to be most favorable. In the 5 configurations 9–11 Cit anions are replaced with Cys AA (Fig. 5). In case of interactions with Glu amino acid on the citrate coated gold slab, the most favorable orientation is when the both the (–COO−) groups are oriented towards the gold slab. In this case 5–8 Cit are replaced by the Glu amino acid residues (Fig. 6). Our simulations with Arg AA on the citrate-capped gold surface suggest the mechanism by which Arg is preferentially adsorbed is via its (–COO−) group towards the Au slab. However, on average only 2–3 citrate anions are replaced with Arg AA. The rest of the AA are contained in the solution (Fig. 7). In the MD simulations we have tested configuration with only 12 AA and 12 citrate anions. It might be possible that this configuration is metastable. Taking, this limitation into account, we have carried out the energetic analysis to find the adsorption energy of different amino-acids on the Au slab. The average energy of the three independent systems (Au, water, Cit and each AA) is obtained from the last 1 ns of the unrestrained MD simulations. The adsorption energy is predicted by calculating the difference in the total potential energy of the last 1 ns of the unrestrained MD simulations and the energy of the starting configurations of three AA on the citrate capped gold slab. The energy difference is favorable for the Cys followed by the Glu and least for the Arg amino acid (Fig. 8).Hemolysis
Although all the three selected AA are nontoxic and frequently used in the body as a source of protein, hormones and enzymes. But, the applications of Cys, Arg and Glu coated AuNPs as drug delivery vehicles require a proper understanding of their hemolytic behavior. Hemolysis is a process in which foreign substances can interact and rupture the RBCs cell membrane and release hemoglobin.45 A similar mechanism is expected if NPs interact with RBCs in the bloodstream when they are planned for use in various biological applications that might require their intravenously administrated formulations. Uncoated NPs either be metal or nonmetal are highly toxic and show strong hemolysis.46,47 Uncoated AuNPs also have been found to have significant interactions with the cell wall of the blood cell, which causes hemolysis.48 The mechanism behind the hemolysis is ascribed to the fact that the RBC membrane consists of three layers with glycocalyx on the exterior, protein network on the anterior, and lipid bilayer in between the two. The glycoprotein49 and lipid bilayers50 are highly susceptible to hydrophilic and hydrophobic interactions and thus are responsible for the rupturing of the cell membranes. In addition to this, agglutination of RBC and to a lesser extent, cell shape deformation has also been detected. Moreover, RBC-bare nanoparticle interaction also increases the circulation time.51 Therefore, this specific internalization mechanism can be modulated by physicochemical properties of the NPs including size, shape, surface charge and surface chemistry etc. Hence to circumvent hemolysis, the AuNPs are properly coated with AA that can be used as drug release vehicles in the systemic circulation for biomedical applications. For this purpose, AA-AuNPs with concentrations 25 μg ml−1 and 50 μg ml−1 are subjected to hemolysis (Fig. 9) to identify minimum concentration that provides minimum hemolysis and hence is best suited for the drug delivery vehicles in the systematic circulation. The percentage hemolysis [(sample absorbance − negative control absorbance)/(positive control absorbance − negative control absorbance) × 100] for AA-AuNPs at two dose concentration of 25 μg ml−1 and 50 μg ml−1 for Cys-AuNPs is 0.83 and 2.07, for Glu-AuNPs is 0.81 and 2.10 and for Arg-AuNPs is 0.29 and 0.98, respectively as depicted in Table 1. The hemolysis increases with the increase in the amount of NPs for all mixtures because greater number of NPs have great potential to break the cell wall, hence it is minimum for 25 μg ml−1 and decreases with decrease in concentration of AA-AuNPs, which can be used as drug release vehicle in the systemic circulation. Lack of marked hemolysis for these samples is related to the presence of proteins along with the RBCs in blood plasma. Because NPs surface is passivated by AA coating, therefore NPs surface never comes in contact with RBCs. AA coating has no adverse effect on the cell membrane. Thus, AA-AuNPs significantly reduce the hemolytic effect in comparison to uncoated NPs especially Arg-AuNPs. | ||
| Fig. 9 Hemolytic absorbance spectra of AA-AuNPs (a) Cys-AuNPs (b) Glu-AuNPs (c) Arg-AuNPs (d) % age hemolysis of AuNPs with different doses of AA-AuNPs. | ||
| AA-AuNPs | Absorbance@540 nm | % hemolysis | ||||||
|---|---|---|---|---|---|---|---|---|
| 25 μg ml−1 | 50 μg ml−1 | −ve control | +ve control | 25 μg ml−1 | 50 μg ml−1 | −ve control | +ve control | |
| Cys-AuNPs | 0.081 | 0.132 | 0.051 | 3.956 | 0.83% | 2.07% | — | 100% |
| Glu-AuNPs | 0.081 | 0.13 | 0.05 | 3.8480 | 0.81% | 2.10% | — | 100% |
| Arg-AuNPs | 0.063 | 0.037 | 3.8480 | 0.074 | 0.29% | 0.98% | — | 100% |
Conclusions
The present study serves a dual purpose by providing synthesis, physiochemical aspects supported by computational studies and biomedical applications of AA coated AuNPs. It concludes that AA having functionalities –SH, –NH2 and –COO− are used to modify the surface of AuNPs at different pH values (3.5, 5, 7, 9 and 11). Ideal physiological conditions are established for AuNPs to be stable and the detailed aggregation kinetics is studied. Different parameters like pH of the media, time, and the nature of the capping ligands are responsible for the stability of AuNPs. Cys undergoes strong and thermodynamically favorable covalent bond formation with Au surface because of the presence of –SH group, independent of pH. It may allow desorption of Cit from AuNPs leading to different extent of aggregation depending upon pH of the media. No aggregation is observed in the case of Glu-AuNPs and Arg-AuNPs because of weaker covalent interactions of –NH2 group with Cit-AuNPs. Thus, AA shows remarkable surface adsorption, which is pH responsive because of their amphiphilic nature. From MD simulations we conclude that the AA, Cys is the most effective in replacing the Cit form the Au slab with the –SH group as the most preferable orientation to interact with the Au surface. Our simulations also suggest that Glu also decisively replaces Cit with both the (–COO−) preferentially adsorbed on the Au. The AA, Arg is least effective in replacing the Cit. Our findings help us in selecting the AA which can selectively displace Cit by noncovalent displacement. Further, these AA-AuNPs are used for observing their hemolytic behavior, where all AA coated AuNPs show little hemolysis with Arg-AuNPs having least hemolytic effect. Thus, this study opens up several possibilities for AA coated AuNPs to be used in various biomedical applications, which require their intravenous administration.Acknowledgements
The authors thank CSIR, New Delhi (No. 01(2700)/12/EMR-II) for providing financial support.References
- R. Maheshwari and A. Dhathathreyan, J. Colloid Interface Sci., 2004, 277, 79–83 CrossRef CAS PubMed.
- A. R. K. Selvaraj, N. A. Murugan and H. Agren, J. Phys. Chem. A, 2012, 116, 11702–11708 CrossRef CAS PubMed.
- R. J. Kassner and W. Yang, J. Am. Chem. Soc., 1977, 99, 4351–4355 CrossRef CAS PubMed.
- M. Gupta, E. F. da Silva and H. F. Svendsen, Energy Procedia, 2014, 51, 161–168 CrossRef CAS.
- V. Bartova, J. Barta, A. Brabcova, Z. Zdrahal and V. Horackova, J. Food Compos. Anal., 2015, 40, 78–85 CrossRef CAS.
- V. Ravindran, M. Abdollahi and S. Bootwalla, Anim. Feed Sci. Technol., 2014, 197, 233–240 CrossRef CAS.
- D. Y. Boudko, J. Insect Physiol., 2012, 58, 433–449 CrossRef CAS PubMed.
- J. Wang, Z. Liu, Y. Wang, W. Cheng and H. Mou, J. Biotechnol., 2014, 187, 34–42 CrossRef CAS PubMed.
- T. G. Cerdan, R. Lopez, J. Portu, L. G. Arenzana, I. L. Alfaro and P. Santamaria, Food Chem., 2014, 163, 136–141 CrossRef PubMed.
- B. A. Boughton, P. Reddy, M. P. Boland, U. Roessner and P. Yates, Food Chem., 2015, 179, 109–115 CrossRef CAS PubMed.
- B. H. Kim, H. S. Lee, Y. A. Jang, J. Y. Lee, Y. J. Cho and C. Kim, J. Food Compos. Anal., 2009, 22, 44–52 CrossRef CAS.
- Y. A. Lin, A. G. Cheetham, P. Zhang, Y. C. Ou, Y. Li, G. Liu, D. H. Merino, I. W. Hamley and H. Cui, ACS Nano, 2014, 8, 12690–12700 CrossRef CAS PubMed.
- L. H. Jones, A. Narayanan and E. C. Hett, Mol. BioSyst., 2014, 10, 952–969 RSC.
- D. Das, G. Roy and G. Mugesh, J. Med. Chem., 2008, 51, 7313–7317 CrossRef CAS PubMed.
- C. Deng, J. Wu, R. Cheng, F. Meng, H. A. Klok and Z. Zhong, Prog. Polym. Sci., 2014, 39, 330–364 CrossRef CAS.
- V. A. Soloshonok, T. Yamada, H. Ueki, A. M. Moore, T. K. Cook, K. L. Arbogast, A. V. Soloshonok, C. H. Martin and Y. Ohfune, Tetrahedron, 2006, 62, 6412–6419 CrossRef CAS.
- C. Chen, H. Shi and G. Zhao, J. Phys. Chem. C, 2014, 118, 12041–12049 CAS.
- A. Datt, N. Ndiege and S. C. Larsen, Nanomaterials for Biomedicine, 2012, 1119, 239–258 CAS.
- K. M. Koczkur, S. Mourdikoudis, L. Polavarapu and S. E. Skrabalak, Dalton Trans., 2015, 44, 17883–17905 RSC.
- L. Polavarapu, S. Mourdikoudis, I. Pastoriza-Santos and J. Pérez-Juste, CrystEngComm, 2015, 17, 3727–3762 RSC.
- O. S. Muddineti, B. Ghosh and S. Biswas, Int. J. Pharm., 2015, 484, 252–267 CrossRef CAS PubMed.
- V. Singh, P. Khullar, P. N. Dave, G. Kaur and M. S. Bakshi, ACS Sustainable Chem. Eng., 2013, 1, 1417–1431 CrossRef CAS.
- N. G. Bastus, J. Comenge and V. Puntes, Langmuir, 2011, 27, 11098–11105 CrossRef CAS PubMed.
- F. Christian, U. Chiara, I. G. Matthew, E. U. Ronald, B. W. Babette, A. R. Ignacio, C. Pierre-Olivier and C. James Kirkpatrick, Part. Fibre Toxicol., 2012, 9, 23 CrossRef PubMed.
- M. Sethi and M. R. Knecht, ACS Appl. Mater. Interfaces, 2009, 1, 1270–1278 CAS.
- M. Sethi and M. R. Knecht, Langmuir, 2010, 26, 9860–9874 CrossRef CAS PubMed.
- X. Zhang, M. R. Servos and J. Liu, Langmuir, 2012, 28, 3896–3902 CrossRef CAS PubMed.
- B. Sharma and M. K. Rabinal, J. Alloys Compd., 2015, 649, 11–18 CrossRef CAS.
- M. Annadhasan, T. Muthukumarasamyvel, V. R. S. Babu and N. Rajendiran, ACS Sustainable Chem. Eng., 2014, 2, 887–896 CrossRef CAS.
- Y. Liu, L. Qiao, L. Liu and R. Guo, Colloids Surf., A, 2015, 474, 92–100 CrossRef CAS.
- C. B. Grosan, C. Varodi, A. Vulcu, L. Olenic, S. Pruneanu and V. Almasan, Electrochim. Acta, 2012, 63, 146–152 CrossRef CAS.
- P. A. Mirau, R. R. Naik and P. Gehring, J. Am. Chem. Soc., 2011, 133, 18243–18248 CrossRef CAS PubMed.
- S. Ju and W. S. Yeo, Nanotechnology, 2012, 23, 135701–135708 CrossRef PubMed.
- G. Brancolini, D. B. Kokh, L. Calzolai, R. C. Wade and S. Corni, ACS Nano, 2012, 6, 9863–9878 CrossRef CAS PubMed.
- I. O. Jiemenez, F. M. Romero, N. G. Bastus and V. Puntes, J. Phys. Chem. C, 2010, 114, 1800–1804 Search PubMed.
- B. Hess, C. Kutzner, D. van der Spoel and E. Lindahl, J. Chem. Theory Comput., 2008, 4, 435–447 CrossRef CAS PubMed.
- M. Pekka and L. Nilsson, J. Phys. Chem. A, 2001, 105, 9954–9960 CrossRef.
- G. Bertrand, J. Mol. Liq., 2002, 101, 219–260 CrossRef.
- F. Iori and S. Corni, J. Comput. Chem., 2008, 29, 1656–1666 CrossRef CAS PubMed.
- F. Iori, R. Di Felice, E. Molinari and S. Corni, J. Comput. Chem., 2009, 30, 1465–1476 CrossRef CAS PubMed.
- K. Okitsu, K. Sharyo and R. Nishimura, Langmuir, 2009, 25, 7786–7790 CrossRef CAS PubMed.
- M. Johnsson, A. Wagenaar and J. B. F. N. Engberts, J. Am. Chem. Soc., 2003, 125, 757–760 CrossRef CAS PubMed.
- D. R. Lide, CRC handbook of chemistry and physics, Boca Raton, Florida, 75th edn, 1994 Search PubMed.
- S. Kumar and S. B. Rai, Indian J. Pure Appl. Phys., 2010, 48, 251–255 CAS.
- M. K. Goshisht, L. Moudgil, P. Khullar, G. Singh, A. Kaura, H. Kumar, G. Kaur and M. S. Bakshi, ACS Sustainable Chem. Eng., 2015, 3, 3175–3187 CrossRef CAS.
- Y. S. Lin and C. L. Haynes, J. Am. Chem. Soc., 2010, 132, 4834–4842 CrossRef CAS PubMed.
- Y. Liu, Z. Zhang, Q. Zhang, G. L. Baker and R. M. Worden, Biochim. Biophys. Acta, 2014, 1838, 429–437 CrossRef CAS PubMed.
- A. Mahal, M. K. Goshisht, P. Khullar, H. Kumar, N. Singh, G. Kaur and M. S. Bakshi, International Journal of Research in Physical Chemistry and Chemical Physics, 2014, 16, 14257–14270 RSC.
- A. Dedinaite, M. Lundin, L. Macakova and T. Auletta, Langmuir, 2005, 21, 9502–9509 CrossRef CAS PubMed.
- J. Cocquyt, U. Olsson, G. Olofsson and P. Van der Meeren, Langmuir, 2004, 20, 3906–3912 CrossRef CAS PubMed.
- T. Mocan, Biotechnol., Mol. Biol. Nanomed., 2013, 1, 7–12 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26502a |
| This journal is © The Royal Society of Chemistry 2016 |