Progress in the production of biomass-to-liquid biofuels to decarbonize the transport sector – prospects and challenges
Abstract
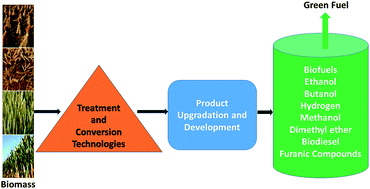
Annually the transport sector consumes a quarter of global primary energy and is responsible for related greenhouse gas emissions. Presently, petroleum derived liquid fuels are the overwhelming source of energy for the transport sector. Liquid biofuels are a viable substitution for petroleum-derived fuels in the transport sector and an important option to mitigate greenhouse gas emissions, especially CO2 emissions. Substituting petroleum-derived fuels with liquid biofuel is also anticipated to reduce the dependency of the transport sector on fossil fuels. Different options are available for the production liquid biofuels. However, the production of liquid biofuels from lignocellulosic biomass has certain advantages. These advantages include the high abundance, availability, low procurement cost and current under-utilization of lignocellulosic biomass. However, the potential for successful deployment of technologies to produce liquid biofuel from lignocellulosic biomass and their cost reductions are surrounded by large uncertainties. High cost of production of liquid fuels from lignocellulosic biomass and their commercial immaturity are major obstacles for the widespread application of liquid biofuels in transportation. Other obstacles include the lack of infrastructure and lack of political as well as public support. This article reviews the obstacles behind the limited production of biomass to liquid (BTL) fuels and their diffusion in the transport sector. The potential approaches to make the production of lignocellulosic-based liquid biofuels economically attractive are also discussed. An approach that focuses on integrating individual operations and processes and adequately modelling these processes evaluated on the bases of the entire pathway can help in realizing the large scale commercial production of liquid biofuels through cleaner production.

 Please wait while we load your content...
Please wait while we load your content...