Using an innovative quality-by-design approach for the development of a stability-indicating UPLC/Q-TOF-ESI-MS/MS method for stressed degradation products of imatinib mesylate
Zhihui Ren,
Xiaoxi Zhang,
Haiyuan Wang and
Xinghua Jin*
School of Pharmaceutical Science and Technology, Tianjin University, Weijin Road 92, Nankai District, Tianjin, 300072, China. E-mail: huaxingjin@gmail.com; Tel: +86 22 27403177
First published on 21st January 2016
Abstract
A stability-indicating UPLC/Q-TOF-ESI-MS/MS method has been developed for the simultaneous determination of imatinib mesylate (IMM) and its impurity and degradation products in the active pharmaceutical ingredient (API) and drug products. Gradient elution of 0.1% g mL−1 ammonium formate buffer at pH 8.57 and acetonitrile were used as the mobile phase and a Waters Acquity UPLC CSH C18, 100 mm × 2.1 mm, 1.7 μm particle size column was utilized as the stationary phase. Forced degradation, such as acid and base hydrolysis, and oxidative stress conditions of IMM were carried out to prove the stability-indicating performance of the method. The quality-by-design (QbD) principle was applied to the method development approach and the chromatography modeling software DryLab®2000 plus and Design Expert®8.0.6 were used to optimize the chromatographic method. The robustness study (Design Space) was performed by varying three critical chromatographic parameters (flow rate, temperature, and pH) at 3 levels (+1, 0, and −1). The result showed that baseline separation of all peaks of IMM and its impurity and degradation products could be achieved and a resolution of 2.0 could be reached in all experiments. The UPLC method was validated for specificity, linearity, accuracy, precision and robustness in compliance with the ICH guideline Q2 (R1). The method we developed was a fast, robust and reliable UPLC method with higher suitability and specificity. Furthermore, elemental composition and major fragments of the impurity and degradation products of IMM were characterized through the optimized UPLC/Q-TOF-MS/MS analysis.
1. Introduction
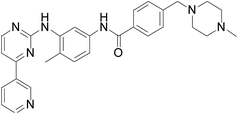
Imatinib mesylate (IMM) is a protein tyrosine kinase inhibitor. As an anticancer drug, it was often used to treat patients with Philadelphia positive chronic myeloid leukemia, chronic myeloid leukemia (CML) in blast crisis, gastrointestinal stromal tumors (GISTs), acute lymphoblastic leukemia and aggressive systemic mastocytosis. The drug was developed by the Swedish company Novartis.1Imatinib mesylate was designated chemically as 4-[(4-methyl-1-piperazinyl) methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide methanesulfonate (Fig. 1).
At present, there is no international pharmacopoeia standard for the estimation of related substances and determination of IMM in the active pharmaceutical ingredient (API) and pharmaceutical formulations. Only several HPLC and UPLC procedures for the determination of IMM and its related substances have been reported in the literature.1,2 Vivekanand et al.3 showed a validated HPLC method for IMM. Moreover, some RP-HPLC methods were used for estimation of IMM and its impurities.4,5 Although HPLC is widely used to analyze the impurities in the API and pharmaceutical formulations, it also has some disadvantages, such as longer analysis time and larger consumption of organic solvents. UPLC as a new technique shows better separation capabilities, shorter separation time and less solvent consumption.1
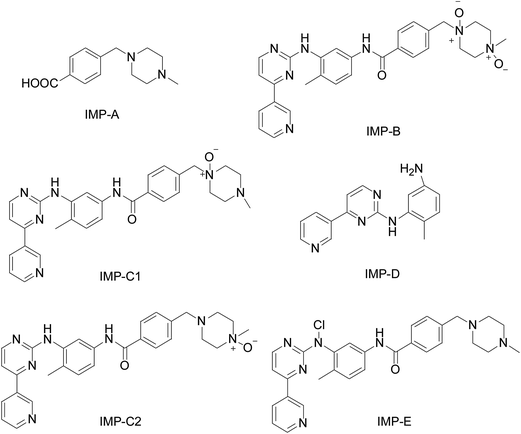
Nowadays, establishment of a validated stability-indicating assay method for the determination of related substances and IMM in the API and drug products is recommended by the International Conference on Harmonization (ICH) guidelines6 and the United State Pharmacopoeia (USP).7 Of these above methods, only one1 is stressed to be a stability-indicating analytical method for IMM and its impurities and degradation products. As an important part of the process of drug product development, a stability test contributes to identifying the degradation products and impurities for the development of a stability-indicating method. Many environmental conditions significantly influence drug stability, such as heat, light, hydrolysis and oxidation. The degradation products of IMM under hydrolytic, oxidation conditions have been reported in the literature.1,2 In this paper, the impurity and degradation products, namely Imp-A, B, C1, C2, D and E, are shown in Fig. 2.8,9
Hence, it is necessary to develop a simple, fast and reliable stability-indicating UPLC method for the determination of IMM and its impurity and degradation products in the API and pharmaceutical formulations.
In the past years, a trial-and-error approach was often used for the development of a chromatographic method, such as the one-factor-at-a-time (OFAT) method. The principle, which requires a great number of experiments to develop a relatively proper method, has many shortcomings, such as being time-consuming, solvent-wasting and having some unknown factors.10 Nowadays, experimental design principles have been widely and successfully used for method development in the pharmaceutical industry. In our study, a small number of experiments were needed to achieve an ideal result based on the chromatography modeling software DryLab®2000 plus which was used for UPLC method development. At the same time, the quality-by-design (QbD) principle, which is recommended by the ICH guideline Q8 (R2)11 and widely requested in pharmaceutical development,12 was applied to build a more scientific and risk-based multifactorial approach. The software Design Expert®8.0.6 was used in the robustness study which is a fundamental criteria of quality in a HPLC method.13 The optimum regions in which it had the best separation of each peak could be found out by the application of the software. Hence, a new stability-indicating UPLC/Q-TOF-ESI-MS/MS method was developed for the determination of IMM and its impurity and degradation products in the API and drug products.14 Moreover, elemental composition and major fragments of the impurity and degradation products of IMM were characterized through the optimized UPLC/Q-TOF-MS/MS analysis.15,16
2. Experimental
2.1. UPLC equipment and chromatographic conditions
LC-MS/MS analysis was carried out on an Agilent 1290 series UPLC instrument (Agilent Technologies, USA) coupled to a quadrupole time-of-flight mass spectrometer (Bruker micrOTOF-QII, Germany) equipped with an electrospray ionization source. Two different columns, a Waters Acquity UPLC CSH C18 column (100 × 2.10 mm i.d., particle size 1.7 μm) and a Phenomenex kinetex C18 column (100 × 2.10 mm i.d., particle size 1.7 μm), were used in the study.All chromatographic experiments were performed in a gradient mode. Solvent A was 0.1% g mL−1 ammonium formate buffer at pH 8.57 (adjusted with ammonia solution) and solvent B was acetonitrile. The flow rate was set to 0.45 mL min−1 and the injection volume was 2 μL. The temperature in the screening experiments was at 41.88 °C. UV detection was carried out at 269 nm. The gradient analysis was as follows: (T/%B) 0/5; 1/15; 1.1/25; 5/25; 5.1/40; 7/48; 9/48.
2.2. Drug and reagents
The API of IMM and impurities (standard substances, purity 99.00%) was provided by Tianjin Taipu pharmaceutical technology development Co., Ltd. Acetonitrile and methanol were HPLC grade, and were purchased from Tianjin KangKeDe Chemical Reagent Co., Ltd. Ammonium formate, ammonia solution, sodium hydroxide, hydrochloric acid and hydrogen peroxide were analytical grade, and all solvents were purchased from Tianjin Jiangtian Chemical Reagent Co., Ltd. High purity water was purified by a Millipore Milli-Q academic water purification system (Tianjin On-well Scientific Co., Ltd).2.3. Software
The chromatography modeling software DryLab®2000 plus (Molnar-Institute, Berlin, Germany) was used for the optimization of gradient time, temperature and pH to separate a mixture of IMM and its impurity and degradation products. The robustness evaluation was performed by Design Expert®8.0.6 (Stat-Ease, Inc., Minneapolis, MN).2.4. Preparation of drug product solution
A standard stock solution containing IMM and all six impurities was prepared with a mixer of MeOH and CH3CN (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v). The sample solution was prepared by dissolving the API of 5 mg IMM substance in a 10 mL volumetric flask with a mixer of MeOH and CH3CN (60
40, v/v). The sample solution was prepared by dissolving the API of 5 mg IMM substance in a 10 mL volumetric flask with a mixer of MeOH and CH3CN (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v), diluted to obtain a concentration of 0.5 mg mL−1. The resulting solution was filtered through a 0.22 μm nylon filter. This clear solution was used for the UPLC determination.
40, v/v), diluted to obtain a concentration of 0.5 mg mL−1. The resulting solution was filtered through a 0.22 μm nylon filter. This clear solution was used for the UPLC determination.
2.5. Stress studies
In the stress studies, the 0.5 mg mL−1 concentration of IMM drug substance was used to provide an indication of the stability-indicating property and specificity of the proposed method. Degradation was attempted under stress conditions of acid (1.0 M HCl at 60 °C, 6 h), base (1.0 M NaOH at 60 °C, 6 h), and oxidation (1% H2O2 at 60 °C, 1 h) to evaluate the ability of the proposed method to separate IMM from its impurity and degradation products. All of the stress conditions were done in the dark. An UV detector (UPLC) and Q-TOF-ESI-MS/MS were used to check the peak purity of the stressed sample.3. Results and discussion
3.1. Design of experiments
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) at a flow rate of 0.5 mL min−1 and the temperature was 35 °C. The results showed that the separation and peak shape of each peak in the mixed sample were not satisfactory.
5) at a flow rate of 0.5 mL min−1 and the temperature was 35 °C. The results showed that the separation and peak shape of each peak in the mixed sample were not satisfactory.So, a series of trials were done by changing the pH of the buffer solution (pH values of 3.0, 6.0 and 9.0), the flow rates (0.4 mL min−1, 0.5 mL min−1, and 0.6 mL min−1), the detection wave length (237 nm, 254 nm, and 269 nm) and the stationary phase [Waters Acquity UPLC CSH C18 column (100 × 2.10 mm i.d., particle size 1.7 μm), Phenomenex kinetex C18 column (100 × 2.10 mm i.d., particle size 1.7 μm)]. After those kinds of trials, the preliminary UPLC method was obtained: on the Waters Acquity UPLC CSH C18 column at 35 °C using 0.1% g mL−1 ammonium formate buffer and acetonitrile in a gradient mode (the initial ratio was 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) at a flow rate of 0.5 mL min−1 and the UV detection was carried out at 269 nm. The choice of the buffer solution, which could also be used for the LC-MS study, expanded its applicability.
5) at a flow rate of 0.5 mL min−1 and the UV detection was carried out at 269 nm. The choice of the buffer solution, which could also be used for the LC-MS study, expanded its applicability.
In positive ion mode; drying gas temperature: 200 °C; drying gas flow: 6.0 L min−1; nebulizer: 1.8 bar; capillary voltage: 4500 V; and scanning range: 50–1000 m/z.
Voltage parameters: transfer: funnel 1 RF: 200 Vpp; funnel 2 RF: 300 Vpp; hexapole RF: 300 Vpp; quadrupole: ion energy: 6 eV; low mass: 200 m/z; collision cell: collision energy: 10 eV; collision RF: 300 Vpp; transfer time: 50 μs; pre-pulse storage: 10 μs.
3.2. UPLC method optimization
 | ||
| Fig. 3 (A) Gradient time–temperature model and (B) gradient time–pH model for the same sample as described. | ||
| (A) | |||||
|---|---|---|---|---|---|
| # | Name | tR (min) | Area | Tail | Rs |
| 1 | Imp-A | 1.00 | 15.1 | 1.00 | 44.09 |
| 2 | Imp-B | 3.08 | 1680.2 | 1.00 | 7.89 |
| 3 | Imp-C1 | 3.7 | 721.1 | 1.00 | 7.85 |
| 4 | Imp-D | 4.44 | 24.8 | 1.00 | 3.67 |
| 5 | Imp-C2 | 4.83 | 416.2 | 1.00 | 13.62 |
| 6 | IMM | 6.59 | 3123.4 | 1.00 | 2.88 |
| 7 | Imp-E | 7.02 | 31.4 | 1.00 | |
| (B) | |||||
|---|---|---|---|---|---|
| # | Name | tR (min) | Area | Tail | Rs |
| 1 | Imp-A | 0.87 | 33.3 | 1.00 | 61.97 |
| 2 | Imp-B | 3.72 | 6197.3 | 1.00 | 10.89 |
| 3 | Imp-C1 | 4.61 | 1324.0 | 1.00 | 19.96 |
| 4 | Imp-C2 | 6.72 | 1502.7 | 1.00 | 14.43 |
| 5 | Imp-D | 7.49 | 370.0 | 1.00 | 3.27 |
| 6 | IMM | 8.69 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 246.7 246.7 |
1.00 | |
3.3. Multifactorial robustness study
| Factor name | Three levels | ||
|---|---|---|---|
| Low (−1) | Middle (0) | High (+1) | |
| T (°C) | 37 | 42 | 47 |
| v (mL min−1) | 0.45 | 0.50 | 0.55 |
| pH | 7.0 | 8.0 | 9.0 |
| Rs1 (Imp-B and Imp-C1) = 2.99 − 0.057A + 0.18C + 0.27AC + 0.21A2 + 0.59C2 | (1) |
| Rs2 (Imp-D and Imp-C2) = 1.71 − 0.084A + 0.060B − 0.13C − 0.46AC − 0.36BC | (2) |
| Rs3 (IMM and Imp-E) = 2.46 − 0.29A + 0.071B + 1.17C + 0.30BC + 0.20A2 + 1.01C2 | (3) |
| TIMM = 0.97 + 0.053A − 0.011B − 0.089C − 0.14A2 + 0.13B2 + 0.98C2 | (4) |
Pareto analysis of variance analysis (ANOVA) and regression analysis were used to test the fitness of the models. The results in Table 6 point out that the equation sufficiently presented the relationship between the input parameters and the response variables. ANOVA is a statistical technique that subdivides the total variation in a data set into component parts combined with sources of variation on the variables of the model.19 The ANOVA results in Table 3 show the F-value for Rs1 (Imp-B and Imp-C1), Rs2 (Imp-D and Imp-C2), Rs3 (IMM and Imp-E) and TIMM as 8.08, 1.82, 60.07 and 238.76 respectively, implying that the model was highly significant. The p-values that were lower than 0.05 indicated that the quadratic model was statistically significant.20
| Source | Coefficient estimate | Sum of squares | DF | Standard error | Mean square | F-value | p-value |
|---|---|---|---|---|---|---|---|
| Rs1 (Imp-B and Imp-C1) | |||||||
| Model | 2.29 | 5 | 0.46 | 8.08 | 0.0020 | ||
| A-v | −0.057 | 0.026 | 1 | 0.084 | 0.026 | 0.47 | 0.5089 |
| C-pH | 0.18 | 0.27 | 1 | 0.084 | 0.27 | 4.70 | 0.0531 |
| AC | 0.27 | 0.28 | 1 | 0.12 | 0.28 | 4.95 | 0.0480 |
| A2 | 0.21 | 0.19 | 1 | 0.12 | 0.19 | 3.28 | 0.0974 |
| C2 | 0.59 | 1.47 | 1 | 0.12 | 1.47 | 25.90 | 0.0003 |
| Residual | 0.62 | 11 | 0.057 | ||||
| Lack of fit | 0.57 | 7 | 0.082 | 6.63 | 0.0433 | ||
| Mean | 3.37 | ||||||
| C.V.% | 7.08 | ||||||
| Adeq precision | 8.409 | ||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Rs2 (Imp-D and Imp-C2) | |||||||
| Model | 1.58 | 5 | 0.32 | 1.82 | 0.1888 | ||
| A-v | −0.084 | 0.056 | 1 | 0.15 | 0.056 | 0.32 | 0.5808 |
| B-T | 0.060 | 0.029 | 1 | 0.15 | 0.029 | 0.17 | 0.6914 |
| C-pH | −0.13 | 0.14 | 1 | 0.15 | 0.14 | 0.79 | 0.3917 |
| AC | −0.46 | 0.85 | 1 | 0.21 | 0.85 | 4.88 | 0.0493 |
| BC | −0.36 | 0.51 | 1 | 0.21 | 0.51 | 2.95 | 0.1139 |
| Residual | 1.91 | 11 | 0.17 | ||||
| Lack of fit | 1.80 | 7 | 0.26 | 9.89 | 0.0214 | ||
| Mean | 1.71 | ||||||
| C.V.% | 4.40 | ||||||
| Adeq precision | 4.947 | ||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Rs3 (IMM and Imp-E) | |||||||
| Model | 16.63 | 6 | 2.77 | 60.07 | <0.0001 | ||
| A-v | −0.29 | 0.69 | 1 | 0.076 | 0.69 | 14.96 | 0.0031 |
| B-T | 0.071 | 0.041 | 1 | 0.076 | 0.041 | 0.88 | 0.3703 |
| C-pH | 1.17 | 10.95 | 1 | 0.076 | 10.95 | 237.34 | <0.0001 |
| BC | 0.30 | 0.35 | 1 | 0.11 | 0.35 | 7.54 | 0.0206 |
| A2 | 0.20 | 0.17 | 1 | 0.10 | 0.17 | 3.79 | 0.0803 |
| C2 | 1.01 | 4.31 | 1 | 0.10 | 4.31 | 93.52 | <0.0001 |
| Residual | 0.46 | 10 | 0.046 | ||||
| Lack of fit | 0.45 | 6 | 0.075 | 26.60 | 0.0035 | ||
| Mean | 3.03 | ||||||
| C.V.% | 7.09 | ||||||
| Adeq precision | 22.207 | ||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| TIMM | |||||||
| Model | 4.28 | 6 | 0.71 | 238.76 | <0.0001 | ||
| A-v | 0.053 | 0.022 | 1 | 0.019 | 0.022 | 7.38 | 0.0217 |
| B-T | −0.011 | 1.012 × 10−3 | 1 | 0.019 | 1.012 × 10−3 | 0.34 | 0.5735 |
| C-pH | −0.089 | 0.063 | 1 | 0.019 | 0.063 | 21.08 | 0.0010 |
| A2 | −0.14 | 0.082 | 1 | 0.027 | 0.082 | 27.41 | 0.0004 |
| B2 | 0.13 | 0.074 | 1 | 0.027 | 0.074 | 24.91 | 0.0005 |
| C2 | 0.98 | 4.03 | 1 | 0.027 | 4.03 | 1347.15 | <0.0001 |
| Residual | 10 | 2.990 × 10−3 | |||||
| Lack of fit | 6 | 4.963 × 10−3 | 165.42 | <0.0001 | |||
| Mean | 1.43 | ||||||
| C.V.% | 3.82 | ||||||
| Adeq precision | 36.518 | ||||||
The fitness of the models was also evaluated by calculation of the coefficient of determination (R2) and the adjusted R2. The values of R2 were calculated to be 0.7961, 0.5146, 0.9866 and 0.9941 for Rs1 (Imp-B and Imp-C1), Rs2 (Imp-D and Imp-C2), Rs3 (IMM and Imp-E) and TIMM respectively, which implied that most of the experimental results were well suited. The high value of the adjusted R2 (0.5339 for Rs1 (Imp-B and Imp-C1), −0.1095 for Rs2 (Imp-D and Imp-C2), 0.9693 for Rs3 (IMM and Imp-E) and 0.9865 for TIMM) supported a high accuracy between the measured and the predicted values. The high coefficient of determination and very small p-value (<0.0001) demonstrated that the quadratic polynomial model was significant and adequate to characterize the actual relationship between the input and response. The coefficient of variation (CV) indicates the scattering of the experimental points from the predicted values of the second order polynomial model.21 The low coefficient of variation value (7.08, 4.40, 7.09 and 3.82) showed a high level of precision and reliability of the experiments conducted.
 | ||
| Fig. 4 Response surface plots (3D) showing the effects of (a) flow rate and temperature, (b) temperature and pH and (c) flow rate and pH on Rs1 (Imp-B and Imp-C1). | ||
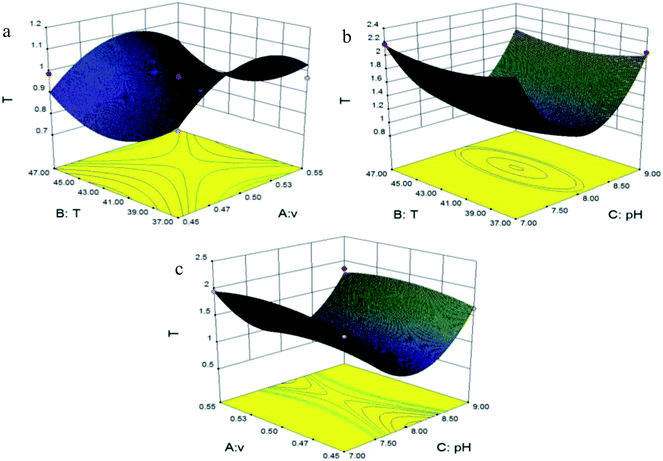
Fig. 4 shows the effects of flow rate, temperature and pH on Rs1 (Imp-B and Imp-C1). Fig. 4(a) depicts that the effect of flow rate and temperature on Rs1 (Imp-B and Imp-C1). Fig. 4(b) illustrates the interaction effect of the independent variables (temperature and pH) on Rs1 (Imp-B and Imp-C1). The response surface plot (Fig. 4(c)) shows that the flow rate and pH also have an effect on Rs1 (Imp-B and Imp-C1). Each parameter showed a significant influence on Rs1 (Imp-B and Imp-C1). In each figure, we found that the value of Rs1 (Imp-B and Imp-C1) increased after a certain degree of decline at the beginning with the increase of flow rate, temperature or pH. However, the best value without considering the other three response parameters couldn’t be found. The same state also happened in the interaction effect of the independent variables (flow rate, temperature and pH) on Rs2 (Imp-D and Imp-C2) (Fig. 5). Fig. 5(a) shows the effect of flow rate and temperature on Rs2 (Imp-D and Imp-C2). The best value of Rs2 (Imp-D and Imp-C2) was located in the edge of the 3D response surface plots (Fig. 5(b) and (c)). Fig. 6 shows the influences of flow rate, temperature and pH on Rs3 (IMM and Imp-E). Fig. 6(a) depicts that Rs3 (IMM and Imp-E) has the most fitness with a low flow rate and high temperature. The response surface plots (Fig. 6(b) and (c)) show the interaction effect of the independent variables on Rs3 (IMM and Imp-E). The variables exhibit a strong influence on Rs3 (IMM and Imp-E). Fig. 7 depicts the effects of flow rate, temperature and pH on TIMM. Fig. 7(a) shows that the flow rate and temperature have a complex effect on TIMM. The response surface plots (Fig. 7(b) and (c)) illustrate that TIMM has the most suitable figure with the interaction effect of the independent variables. In this study, the independent parameters showed a significant influence on the separation efficiency. The maximum value of each response parameter was usually achieved at a relatively high temperature and pH due to the structures of components and the theory of analysis. However, the optimized independent variables in the final method should be obtained by the desired function in the Design Expert®8.0.6 software.
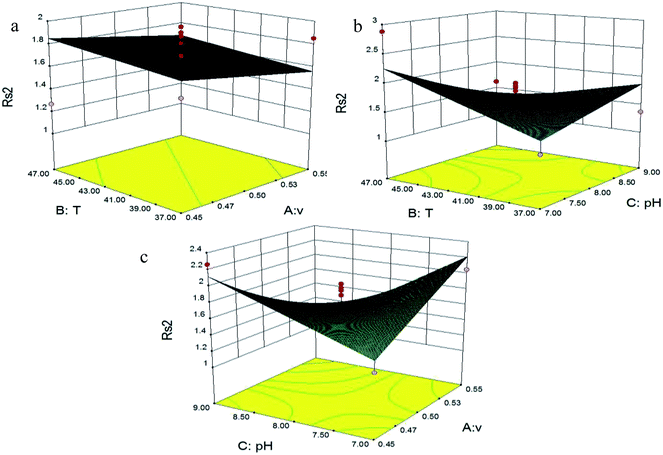 | ||
| Fig. 5 Response surface plots (3D) showing the effects of (a) flow rate and temperature, (b) temperature and pH and (c) flow rate and pH on Rs2 (Imp-D and Imp-C2). | ||
 | ||
| Fig. 6 Response surface plots (3D) showing the effects of (a) flow rate and temperature, (b) temperature and pH and (c) flow rate and pH on Rs3 (IMM and Imp-E). | ||
| Number | Optimal levels of response parameters | |||
|---|---|---|---|---|
| Rs1 | Rs2 | Rs3 | TIMM | |
| 1 | 5.00 | 2.98 | 4.51 | 0.98 |
| 2 | 4.93 | 2.98 | 4.51 | 0.99 |
| 3 | 5.00 | 2.98 | 4.51 | 0.99 |
| 4 | 4.93 | 2.91 | 4.51 | 0.99 |
| 5 | 5.00 | 2.98 | 4.51 | 0.98 |
| 6 | 5.00 | 2.98 | 4.51 | 0.99 |
| RSD% | 0.73% | 0.96% | 0% | 0.52% |
3.4. Method control
A summary of the chromatographic parameters and the final method are given in Table 5. The method was applied for the determination of the impurity and degradation products of IMM in API and pharmaceutical formulations. A typical chromatogram of IMM and all of its impurity and degradation products (A–E) is shown in Fig. 9.| Column | Waters Acquity UPLC®CSH C18, 2.1 mm × 50 mm, 1.7 μm |
| Eluent A | 0.1% g mL−1 ammonium formate buffer (pH 8.57) |
| Eluent B | Acetonitrile |
| Gradient program | (T/%B) 0/5; 1/15; 1.1/25; 5/25; 5.1/40; 7/48; 9/48 |
| Run time | 9 min |
| Flow rate | 0.45 mL min−1 |
| Column temperature | 41.88 °C |
| Injection volume | 2 μL |
| Detection UV | 269 nm |
 | ||
| Fig. 9 Experimental chromatogram of a real sample containing IMM and all of its impurity and degradation products (A–E) for conditions at the optimal values. | ||
Under the developed UPLC conditions, the system suitability parameters were evaluated for IMM and all of its impurity and degradation products. The resolution between any two adjacent components was more than 2.0, and the tailing factor for all the components was between 0.95 and 1.25 (Table 6).
| Compound | RT (min) | RRTa (n = 6) | USP resolutionb (n = 6) | USP tailing factor (n = 6) |
|---|---|---|---|---|
| a Relative retention time (RRT) was calculated against the retention time (RT) of IMM.b Resolutions were calculated between two adjacent peaks. | ||||
| Imp-A | 1.03 | 0.16 ± 0.01 | — | 1.21 ± 0.02 |
| Imp-B | 3.07 | 0.48 ± 0.00 | 9.60 ± 0.01 | 1.04 ± 0.01 |
| Imp-C1 | 3.69 | 0.58 ± 0.00 | 5.00 ± 0.02 | 1.20 ± 0.01 |
| Imp-D | 4.40 | 0.70 ± 0.00 | 3.63 ± 0.01 | 1.19 ± 0.03 |
| Imp-C2 | 4.82 | 0.76 ± 0.01 | 2.98 ± 0.03 | 1.08 ± 0.01 |
| IMM | 6.28 | 1.00 ± 0.01 | 7.16 ± 0.01 | 0.99 ± 0.01 |
| Imp-E | 6.91 | 1.10 ± 0.00 | 4.51 ± 0.00 | 1.13 ± 0.01 |
A validation study was performed in compliance with the ICH guideline Q2 (R1). The validation data are shown in Table 7. In addition, the robustness study of the developed method was carried out to evaluate the effect of small variation in the chromatographic conditions. Three main factors (flow rate (±0.05 mL min−1), pH (±0.5) and column temperature (±5 °C)) were chosen for this study. From the results of all the experiments of the deliberately altered conditions, it can be seen that a resolution between two adjacent peaks of 2.0 can be reached. Therefore, it indicated that the method is robust against small changes of the chromatographic conditions.
| Test | Test details | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Imp-A | Imp-B | Imp-C1 | Imp-D | Imp-C2 | Imp-E | IMM | ||
| Specificity | There are no peaks present in the chromatogram of the sample solvent and eluent at the retention time window of IMM and its known impurities and degradation products | |||||||
| Linearity | 5 concentration points in the range from LOQ to 200% (R2) | 0.99959 | 0.99975 | 0.99998 | 0.99961 | 0.99999 | 0.99972 | 1.0 |
| Limit of detection | Calculated from S/N = 3 | 0.64 μg mL−1 | 1.78 μg mL−1 | 1.63 μg mL−1 | 0.91 μg mL−1 | 0.16 μg mL−1 | 0.96 μg mL−1 | 0.088 μg mL−1 |
| Limit of quantification | Calculated from S/N = 10 | 1.93 μg mL−1 | 5.41 μg mL−1 | 4.93 μg mL−1 | 2.74 μg mL−1 | 0.50 μg mL−1 | 2.91 μg mL−1 | 0.27 μg mL−1 |
| Accuracy | Three different levels (RSD) | 1.03% | 1.85% | 2.40% | 2.56% | 2.63% | 2.70% | 0.27% |
| Precision (repeatability) | n = 6 (RSD) (operator A) | 1.46% | 1.06% | 1.09% | 1.96% | 0.98% | 2.08% | 1.08% |
| Intermediate precision | n = 6 (RSD) (operator B) | 1.73% | 1.11% | 1.37% | 1.78% | 1.25% | 3.09% | 1.36% |
3.5. UPLC-Q-TOF-MS/MS study
In our study, the impurity and degradation products were identified through the optimized UPLC-Q-TOF-MS/MS method. The UPLC-ESI-MS/MS spectra of [M + H]+ ions of IMM and its impurity and degradation products obtained under the stress conditions are given in Fig. 10.A total of eleven components (containing IMM and the impurity and degradation products) were identified and characterized using the Q-TOF-MS/MS experiments. The data from the Q-TOF-MS/MS of IMM and the impurity and degradation products are given in Table 8. Under the conditions of stress studies, two acid degradation products were received. The molecular formulas were C13H18N2O2 ([M + H]+ ion, m/z 235) and C16H15N5 ([M + H]+ ion, m/z 278). Four oxidation degradation products were obtained. The molecular formulas were C29H31N7O2 ([M + H]+ ion, m/z 510), C16H15N5 ([M + H]+ ion, m/z 278), C29H31N7O2 ([M + H]+ ion, m/z 510) and C29H31N7O3 ([M + H]+ ion, m/z 526). Under the conditions of the stress study, IMM also had one impurity which was C29H30N7OCl ([M + H]+ ion, m/z 528). The data of the MS/MS fragment ions of all components shown in Table 8 were used to illustrate the structure of each component which is mentioned in the introduction (Fig. 2).
| Compound | Retention time (min) | Molecular formula | Observed mass m/z | Calculated mass m/z | Error (ppm) | MS/MS fragment ions |
|---|---|---|---|---|---|---|
| Imp-A | 1.2 | C13H19N2O2+ | 235.1504 | 235.1514 | 4.2 | 215, 121, 117, 101 |
| Imp-B | 3.4 | C29H32N7O3+ | 526.2537 | 526.2551 | 2.6 | 394, 378, 277, 247, 222, 194 |
| Imp-C1 | 4.1 | C29H32N7O2+ | 510.2592 | 510.2612 | 3.9 | 394, 380, 277, 262, 222, 194 |
| Imp-D | 4.9 | C16H16N5+ | 278.1423 | 278.1410 | −4.6 | 262, 245, 196, 157 |
| Imp-C2 | 5.4 | C29H32N7O2+ | 510.2595 | 510.2612 | 3.4 | 423, 395, 277, 222 |
| IMM | 6.7 | C29H32N7O+ | 494.2645 | 494.2663 | 3.5 | 394, 379, 264, 247, 222, 194 |
| Imp-E | 7.4 | C29H31ClN7O+ | 528.2236 | 528.2253 | 3.2 | 428, 392, 377, 281, 220, 180 |
4. Conclusions
In this paper, a simple and rapid UPLC-Q-TOF-ESI-MS/MS method used for the determination of IMM and its impurity and degradation products was rationally developed. The resulting method gave a good separation of all 7 peaks of the sample of IMM and its impurity and degradation products with a critical resolution (Rs, crit > 2.0) well above the baseline separation, with a run time of under 9 min.22,23In our study, an innovative quality-by-design approach was used for the development of a stability-indicating, fast, robust and reliable UPLC method. With the assistance of various modeling software programs (DryLab®2000 plus and Design Expert®8.0.6), a great number of experiments were reduced, and highly influential chromatographic parameters were also optimized. Fully considering the factors of separation, analysis time and robustness, an ideal method was developed eventually. In addition, the method was fully validated in compliance with ICH guidelines. The suitability and specificity of the method for the determination of IMM and its impurity and degradation products which were characterized unambiguously using the optimized UPLC-Q-TOF-MS/MS method have been presented in this article.
Acknowledgements
We are thankful to the School of Pharmaceutical Science and Technology (SPST), TianJin university for support with the instruments.References
- A. Nageswari, K. V. S. R. Reddy and K. Mukkanti, J. Pharm. Biomed. Anal., 2012, 66, 109–115 CrossRef CAS PubMed.
- W. J. Szczepek, B. Kosmacińska, A. Bielejewska, W. Łuniewski, M. Skarżyński and D. Rozmarynowska, J. Pharm. Biomed. Anal., 2007, 43, 1682–1691 CrossRef CAS PubMed.
- V. V. Vivekanand, D. Sreenivas Rao, G. Vaidhyanathan, N. M. Sekhar, S. Avijit Kelkar and P. Ramachandra Puranik, J. Pharm. Biomed. Anal., 2003, 33, 879–889 CrossRef CAS PubMed.
- D. Ivanovic, M. Medenica, B. Jancic and A. Malenovic, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 800, 253–258 CrossRef CAS.
- M. Medenica, B. Jancic, D. Ivanovic and A. Malenovic, J. Chromatogr. A, 2004, 1031, 243–248 CrossRef CAS PubMed.
- ICH Q1A (R2), Stability Testing of New Drug Substances and Products, February, 2003.
- US Pharmacopoeia Convention, The United States Pharmacopoeia, MD, 26th edn, 2003, pp. 1151–1154 Search PubMed.
- W. Lihong, H. Yuan, Z. Yanqiao, Z. Sujuan and W. Baowei, Chin. J. Pharm., 2011, 42, 728–731 Search PubMed.
- Z. Sujuan, Y. Lixia, C. Xiaolei, S. Qiannan and L. Min, Chin. J. Pharm. Anal., 2013, 33, 633–637 Search PubMed.
- B. Nickerson, Sample Preparation of Pharmaceutical Dosage Forms, Springer, Heidelberg, 2011 Search PubMed.
- ICH Q8 (R2), Pharmaceutical development, August, 2009.
- FDA, A risk-based approach, MD, Rockville, August, 2002.
- K. Monks, I. Molnár, H. J. Rieger, B. Bogáti and E. Szabó, J. Chromatogr. A, 2012, 1232, 218–230 CrossRef CAS PubMed.
- A. H. Schmidt and I. Molnár, J. Pharm. Biomed. Anal., 2013, 78–79, 65–74 CrossRef CAS PubMed.
- P. N. Patel, D. Rajesh Kumar, S. Gananadhamu and R. Srinivas, RSC Adv., 2015, 5, 21142–21152 RSC.
- P. D. Kalariya, M. Sharma, P. Garg, J. Reddy Thota, S. Ragampeta and M. V. N. Kumar Talluri, RSC Adv., 2015, 5, 31024–31038 RSC.
- R. M. Borkar, B. Raju, R. Srinivas, P. Patel and S. K. Shetty, Biomed. Chromatogr., 2012, 26, 720–736 CrossRef CAS PubMed.
- V. M. Rodríguez-González, A. Femenia, R. Minjares-Fuentes and R. F. González-Laredo, LWT--Food Sci. Technol., 2012, 47, 225–232 CrossRef.
- J. Yanping, W. Yunhai, C. Julin and W. Yunying, J. Taiwan Inst. Chem. Eng., 2014, 45, 589–595 CrossRef.
- S. K. Soni, N. Goyal, J. K. Gupta and R. Soni, Starch - Stärke, 2012, 64, 64–77 CrossRef CAS.
- G. J. Swamy, A. Sangamithra and V. Chandrasekar, Dyes Pigm., 2014, 111, 64–74 CrossRef CAS.
- I. Molnár, H. J. Rieger and K. E. Monks, J. Chromatogr. A, 2010, 1217, 3193–3200 CrossRef PubMed.
- R. M. Bianchini, P. M. Castellano and T. S. Kaufman, Anal. Chim. Acta, 2009, 654, 141–147 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |