Cell-laden alginate/polyacrylamide beads as carriers for stem cell delivery: preparation and characterization
Deepti Ranaa,
Aleya Tabasuma and
Murugan Ramalingam*ab
aCentre for Stem Cell Research (CSCR), A Unit of Institute for Stem Cell Biology and Regenerative Medicine-Bengaluru, Christian Medical College Campus, Vellore 632002, India. E-mail: rmurug2000@gmail.com
bWPI-Advanced Institute for Materials Research, Tohoku University, Sendai 980-8577, Japan
First published on 15th February 2016
Abstract
Stem cell based therapies employ engraftment or systemic administration methods for the delivery of stem cells into the target tissues to enhance their regenerative potential. However, majority of the stem cells were found to migrate away from the target site soon after the transplantation, which directly hinders their clinical efficacy, in particular while treating cartilage defects. Therefore, the present study was designed to explore the feasibility and efficacy of an alginate/polyacrylamide (Algi/PAAm) composite biomaterial in the form of cell-laden hydrogel beads as a suitable carrier system to be able to hold the stem cells at the target site and deliver them efficiently. Human bone marrow-derived mesenchymal stem cells (hBMSCs) have been used as a model cell. The beads prepared at an optimized concentration ratio were characterized to study their physicochemical properties. Furthermore, cell-encapsulated Algi/PAAm beads were evaluated for their biological properties. The result of this study has demonstrated that the Algi/PAAm beads with their optimal composition were able to maintain the viability of the encapsulated cells during the period of study, suggesting the cellular compatibility of the beads. Additionally, the encapsulated cells showed round morphology within the beads, in contrast to the 2D-cultured spindle-like shape of hBMSCs. Based on the experimental data obtained in this study, cell-laden Algi/PAAm beads may serve as a potential carrier system for stem cell delivery.
1. Introduction
Stem cell therapy holds great promise in the treatment of various diseases as well as in the regeneration of damaged or defective tissues. Stem cells have a great capacity to differentiate into a variety of cell types and therefore, stem cell transplantation has been elucidated as an effective therapeutic strategy for the regeneration of the target tissues. Despite the initial promising results obtained from preclinical/clinical studies, stem cell therapy has not been able to be actualized as a routine therapeutic option in the clinic. The major issues attributed to this, could be the poor stem cell retention, engraftment in the hostile environment of the target tissue and the need for an optimized system for delivering sufficient numbers of stem cells.1 These issues have been remained to be resolved before the complete translation of stem cell-based therapies. As a matter of fact, for the functional regeneration of the target tissue, sufficient quantity of the stem cells should be re-delivered to the patient. Stem cells can be transplanted directly into the target site and/or administered peripherally via different delivery techniques. However, the transplanted cells experience a harsh environment and in general, more than 95% of cells die shortly after the transplantation,1 which could be attributed to ischemia due to absence of vascularization and cell attachment sites resulting in cell death (anoikis), host immune response and low cell density.2,3 Furthermore, the conventional injection methods often result in poor cell survival and low levels of cell integration into the host tissue.4To overcome these limitations, stem cell delivery systems have been developed. Stem cell delivery systems are specially designed to provide the cell attachment sites, protect from host immune cells, and localize the cells to achieve appropriate cell densities. Ideally, the system should be non-immunogenic, biodegradable at appropriate rates and stable at physiological pH and temperature. Several biomaterials-based delivery systems have been used for stem cell delivery in the form of nanofibers, microspheres, beads, hydrogels, etc. Each form of carrier system has its own advantages as well as disadvantages depending on the application. For example, nanofiber system has mostly being used as tissue scaffold but its application in the local delivering of stem cells is limited. Similarly, microspheres cannot be used with higher cell densities of encapsulated cells and, furthermore, there is a chance of migration from the implanted site. Among them, hydrogel provides the hydrated environment to the cultured cells but struggles for its mechanical stability.5 Among different types of hydrogels, cell-laden hydrogels have gained much attention for the delivery of stem cells due to their ability to fix stem cells in place while providing a hydrated microenvironment. These systems also provide a means to allow for the factors produced by the encapsulated stem cells to diffuse into the target site.4 Therefore, these by enhancing the mechanical stability of them, these cell-laden hydrogels could be used in the development of a clinically translatable delivery system to improve the effectiveness of the stem cell delivery for various tissue engineering and regenerative medicine applications.
The availability of large quantities of mesenchymal stem cells (MSCs), multilineage differentiation potential, readily propagation in culture and no requirement for adjunctive immunosuppressive therapies,6 has made MSCs the most relevant progenitor cell source for tissue engineering applications, in particular cartilage tissue engineering. MSCs have the ability to migrate and engraft onto sites of injury and undergo site-specific differentiation. Therefore, MSCs have been used readily in direct injection for target tissue repair (for example, cartilage). Although the results of this clinical approach were reassuring due to its minimal invasiveness, but it has been observed that the direct injection of MSCs often results in poor cell viability. This could be attributed to the direct exposure of injected stem cells to the damaging mechanical forces that can injure the cells, thereby emphasizing on the need of an optimized delivery system.1 Additionally, a majority of the MSCs were found to migrate away from the target site soon after the transplantation, which being a crucial process further hinders its clinical efficacy.7 The method and site of delivery most likely affects the trafficking of MSCs to target organs. Therefore, encapsulation system based on stem-cell laden hydrogels (Algi/PAAm, for example) could be used as a suitable system for maintaining the natural cell phenotypes, and for delivering MSCs in situ at the target site. With three-dimensional (3D) scaffolds, cell survival and graft integration can be augmented during cell transplantation.8 Furthermore, 3D scaffolds are required to maintain proper growth, preservation of the morphology and production of natural chondrocyte matrix components.8–11
In view of this, immobilization of MSCs in alginate gels is a well-known technology used for various tissue engineering applications. MSCs immobilized in alginate gels maintain good viability during long-term culture due to the mild environment of the gel network. These alginate gels may function as a protective barrier towards physical stress and to avoid immunological reactions with the host.12 For most of the stem cell delivery applications hydrogel beads have been used to allow diffusion of essential nutrients.13 Although the cell-crosslinked alginate hydrogel beads show excellent bioactivities, the network exhibits low strength and toughness, which may limit its practical applications.12 These limitations of the alginate-based scaffolds could be overcome by the use of the combination of tough polymers such as polyacrylamide (PAAm) in the form of composite cell-laden hydrogel beads which can show potent mechanical and physical stability properties closer to natural human cartilage tissue.14 as well as could prove suitable for the controlled delivery of MSCs in situ.15 PAAm is a biocompatible synthetic polymer and has been clinically proved to be non-toxic, non-immunogenic and stable in human body.16 PAAm is an ingredient in a variety of cosmetic and beauty products, and has been approved by the U.S, Food and Drug Administration (FDA) with limitations in quantity, to be used in food-packaging adhesives, paper and paperboards, to wash or peel fruits and vegetables and in imprinting soft-shell gelatin capsules.17 Keeping the above points in view, in this study, authors report the preparation and characterization of cell-laden Algi/PAAm beads suitable for the delivery of stem cells, in particular hBMSCs, for tissue engineering applications such as cartilage tissue regeneration.
2. Experimental
Materials
For material preparation, acrylamide (Cat. no. 164855000), N,N′-methylenebisacrylamide (Cat. no. 163805000) and N,N′,N′,N′-tetramethylethylenediamine (TEMED) (Cat. no. 138455000) were purchased from Acros Organics, Belgium. Ammonium persulfate (APS) (Cat. no. 15055) and calcium chloride (Cat no. 10043524) was bought from Qualigen India. Sodium alginate (Cat. no. W201502) was obtained from Sigma-Aldrich USA and used as received. For cell culture experiments, MEM alpha modification (1×) medium (Cat. no. SH30265.01) was purchased from HyClone. Fetal bovine serum, (Cat. no. 12003C-500ML) from SAFC Biosciences, and penicillin/streptomycin (Cat. no. 15140-122) and L-glutamine 200 mM (100×) (Cat. no. 25030-081) were obtained from Life Technologies. Dulbecco's Phosphate Buffer Saline (PBS) (Cat. no. D8537) purchased from Sigma-Aldrich, USA. Ficoll-Paque (Cat. no. 17-1440-02) from GE Healthcare, 0.25% trypsin–EDTA solution 1× (Cat. no. TCL014-6X500ML) from Himedia and CryoSure-DMSO (Cat. no. 0482) from WAK-Chemie Medical GmbH received, respectively. For cell staining experiments the live/dead kit (Cat. no. L3224) was purchased from Life Technologies and cell counting kit (CCK-8) (Cat. no. 96992) from Sigma (USA).Isolation and culture of human bone marrow derived mesenchymal stem cells (hBMSCs)
Human bone marrow sample was obtained from donors with their written consent in Christian Medical College and Hospital, India. The following experimental protocol was used for isolation of hBMSCs. Briefly, about 5 ml of bone marrow suspension was harvested from posterior iliac crest of donor and mixed with same volume of heparinized (10 U ml−1) PBS to prevent clotting. The mixture was kept at 4 °C prior to further processing in the laboratory. Small aliquot of 100 μl volume were taken for the monocyte counting. Mononuclear cells (MNCs) were isolated as previously described protocol with some modifications.18 Briefly, bone marrow sample was subsequently loaded on the top with Ficoll-Paque solution in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
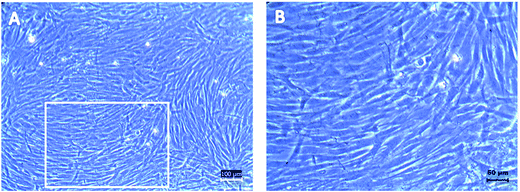
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (bone marrow to Ficoll-Paque ratio) preloaded in a centrifuge tube to form a density gradient, followed by centrifugation at 400g for 30 min at 22 °C. After centrifugation, the yellow plasma layer obtained from the bone marrow sample was discarded and thin MNCs layer was carefully collected and washed twice with 12 ml of PBS, 700 g for 6 min. After washing it twice, the cell pellet was dispersed into 2 ml of α-MEM (10% FBS) media for further seeding and cell counting. The isolated cells were suspended in α-MEM of 440 ml mixed with 5 ml of penicillin/streptomycin (1%), 5 ml of glutamine (1%) and 50 ml of FBS (10%) and seeded into 25 cm2 flasks. Cells were cultured by incubating at 37 °C, 5% CO2. After 3 days, non-adherent cells were removed by washing twice with PBS. Media was changed once in every 3 days. The cell confluence and morphology was monitored under an inverted microscope (CKX41, Olympus). Individual fibroblast-like cells and small colonies of these cells can be seen by inverted microscope after 3 to 4 days. As the cells proliferate the colonies increase in size and become more distinct by 7 days of culture. After 10–14 days of culture the seeded cells can be seen to acquire spindle morphology that seemingly confirms the presence of MSCs. When these primary cultured cells reached 60–70% confluence, they were harvested using 0.25% trypsin/EDTA and sub cultured. The third passage cells were used for subsequent studies. Cells isolated from bone marrow samples at passage 3 were characterized for identification of the phenotypes using flow cytometry and morphology using inverted microscope.
1 ratio (bone marrow to Ficoll-Paque ratio) preloaded in a centrifuge tube to form a density gradient, followed by centrifugation at 400g for 30 min at 22 °C. After centrifugation, the yellow plasma layer obtained from the bone marrow sample was discarded and thin MNCs layer was carefully collected and washed twice with 12 ml of PBS, 700 g for 6 min. After washing it twice, the cell pellet was dispersed into 2 ml of α-MEM (10% FBS) media for further seeding and cell counting. The isolated cells were suspended in α-MEM of 440 ml mixed with 5 ml of penicillin/streptomycin (1%), 5 ml of glutamine (1%) and 50 ml of FBS (10%) and seeded into 25 cm2 flasks. Cells were cultured by incubating at 37 °C, 5% CO2. After 3 days, non-adherent cells were removed by washing twice with PBS. Media was changed once in every 3 days. The cell confluence and morphology was monitored under an inverted microscope (CKX41, Olympus). Individual fibroblast-like cells and small colonies of these cells can be seen by inverted microscope after 3 to 4 days. As the cells proliferate the colonies increase in size and become more distinct by 7 days of culture. After 10–14 days of culture the seeded cells can be seen to acquire spindle morphology that seemingly confirms the presence of MSCs. When these primary cultured cells reached 60–70% confluence, they were harvested using 0.25% trypsin/EDTA and sub cultured. The third passage cells were used for subsequent studies. Cells isolated from bone marrow samples at passage 3 were characterized for identification of the phenotypes using flow cytometry and morphology using inverted microscope.
Synthesis of Algi/PAAm beads
The experimental protocol for synthesis of Algi/PAAm beads is given below. In brief, a master solution of 40% acrylamide and 2% N,N′-methylenebisacrylamide monomers was prepared in deionized (DI) water. Solution was filtered using 0.45 micron filtration flask and kept in dark for storage until further use. 1.5% (w/v) alginate solution was prepared separately in DI water with magnetic stirring. Pre-polymer solutions i.e., acrylamide master solution and alginate solution, were mixed together in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratios, respectively, under constant magnetic stirring of 200 rpm at room temperature (RT), making final volume of 5 ml. This solution was transferred to a 5 ml syringe with a built-in 24 G needle. This pre-polymer solution was subsequently dispensed from the syringe drop by drop into the gel-forming solution i.e., 16 ml of CaCl2 – 102 mM solution mixed with 4 ml of (20% v/v) TEMED with moderate magnetic movement, allowing alginate and acrylamide monomers to completely polymerize (∼10 min) until the beads were completely crosslinked.
5 ratios, respectively, under constant magnetic stirring of 200 rpm at room temperature (RT), making final volume of 5 ml. This solution was transferred to a 5 ml syringe with a built-in 24 G needle. This pre-polymer solution was subsequently dispensed from the syringe drop by drop into the gel-forming solution i.e., 16 ml of CaCl2 – 102 mM solution mixed with 4 ml of (20% v/v) TEMED with moderate magnetic movement, allowing alginate and acrylamide monomers to completely polymerize (∼10 min) until the beads were completely crosslinked.
For comparative analysis, PAAm and alginate beads were also synthesized separately. Briefly, PAAm hydrogels were synthesized by mixing the appropriate volume of the master solution of acrylamide and bisacrylamide with ammonium phosphate (APS) (20 wt%) and TEMED (20% v/v) in the total volume ratio of 1/100 and 1/1000 respectively, under constant stirring. Alginate beads were prepared with the same protocol except for the addition of the acrylamide master solution and TEMED.
Encapsulation of hBMSCs in Algi/PAAm beads
The hBMSCs were encapsulated in Algi/PAAm beads by using the following method. The cells were trypsinized from tissue culture flask, followed by washing in PBS and then isolation of the cell pellet. The isolated hBMSCs at passage 3 were suspended at a seeding density of 1000 cells per cm2 in pre-polymer solution. The cell suspension mixed with pre-polymer solution, was slowly dispensed through a 24 G needle and dropped into the gel-forming solution. The beads with approximately 500 cells per bead (diameter 3 mm) were allowed to polymerize for 10 min and washed twice with DPBS (Fig. 1). The beads were then transferred into 96-well plate with 4 bead in each well filled with medium (α-MEM supplemented with 10% FBS, 1% penicillin/streptomycin and 1% L-glutamine). The beads were cultured at 37 °C in a humidified atmosphere of 5% CO2 for up to 2 day. No dissolution of the beads was observed during the culture period.Characterization
3. Results and discussion
This study demonstrates the synthesis and analysis of the cell-laden Algi/PAAm hydrogel beads in an optimal composition, which could be useful as hBMSCs delivery system for tissue regenerative applications, in particular, cartilage repair. The hypothesis has been supported through the following experimental data.Culture and characterization of hBMSCs
Synthesis and characterization of Algi/PAAm beads
The general gelation mechanism involved in the preparation of cell-laden alginate beads through the ionotropic gelation technique involves extrusion of cell-alginate mixture into CaCl2 solution. By this method, the alginate in the outer layer of the drop which is in immediate contact with calcium ions becomes insoluble and after some time of curing in CaCl2 solution the alginate present inside the droplet also gets converted into calcium alginate due to the diffusion of calcium ions from the bulk external phase.12 Preparation of Algi/PAAm beads involves the dropping of the acrylamide/alginate mixture through a syringe needle into a CaCl2/TEMED solution with continuous stirring at room temperature. Since five parts of alginate is mixed with one part of acrylamide solution, the immediate visible gelation of the bead can be attributed to the ionotropic gelation of the alginate.21 The outer layer of the bead becomes solidified immediately due to the formation of calcium alginate entrapping the acrylamide solution. Meanwhile, it can be proposed that the presence of TEMED in the curing solution could also contribute to the polymerization of the acrylamide, leading to an interpenetrating polymeric network of alginate and polyacrylamide within the beads. The composite system is a novel, biocompatible and fast in situ forming gel bead system in a unique composition ratio of alginate and polyacrylamide. The hydroxyl groups in alginate crosslinks with free amide groups on acrylamide for formulating nontoxic gel beads with highly porous internal structures. The intermolecular hydrogen bonding provides an additional mechanical strength to the cross-linked polymeric gel network. Alginate to PAAm concentration has been optimized so as to minimally affect the cellular compatibilities and also the mechanical stability of the system. The photographic images of prepared Algi/PAAm hydrogel beads are shown in Fig. 4. It can be seen from the figure that the shape of the beads is round and size is ∼3 mm. However, by manipulating the experimental conditions, the size of the beads could be modulated.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration bands of amide and acid groups have been observed at 1703.76 cm−1 and the peak for CH
O stretching vibration bands of amide and acid groups have been observed at 1703.76 cm−1 and the peak for CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 group was observed at 1620.25 cm−1. The FTIR spectra of alginate bead exhibited characteristic broad band at 3362.32 cm−1 corresponding to O–H stretching vibrations of alginate. A sharp peak observed at 1620.90 cm−1 corresponds to carbonyl group of –COO− moiety present in Ca2+ crosslinked alginate polymers. A characteristic carboxylate group peak was also observed at 1463.85 cm−1. In the spectrum of Algi/PAAm beads, the shoulder peaks appeared at 3417.65 cm−1 and a sharp peak at 1673.69 cm−1 corresponding to the N–H stretching and C
CH2 group was observed at 1620.25 cm−1. The FTIR spectra of alginate bead exhibited characteristic broad band at 3362.32 cm−1 corresponding to O–H stretching vibrations of alginate. A sharp peak observed at 1620.90 cm−1 corresponds to carbonyl group of –COO− moiety present in Ca2+ crosslinked alginate polymers. A characteristic carboxylate group peak was also observed at 1463.85 cm−1. In the spectrum of Algi/PAAm beads, the shoulder peaks appeared at 3417.65 cm−1 and a sharp peak at 1673.69 cm−1 corresponding to the N–H stretching and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, respectively, which confirms the crosslinking between the two groups. The presence of a peak at 1434 cm−1 in the Algi/PAAm beads corresponds to the C–N bending vibration, which further supports the crosslinking reaction. The sharp hydroxyl peak of alginate bead at 1620.90 cm−1 was observed to be split and shifted to a higher value of 1673.69 cm−1 and 1625.55 cm−1. The shift in the wavenumber of hydroxyl group of alginate bead may be due to an effect on bond formation involving adjacent hydroxyl groups in alginate and the CONH2 groups in PAAm as a result of the conformational changes in the crosslinked alginate and PAAm. A shift in the sharp peak at 649.07 cm−1 in PAAm to 612.51 cm−1 in Algi/PAAm bead was observed which could be attributed to NH2 bond wagging and bending vibration for the out of the plane carbon. The intermolecular hydrogen bonding provides an additional mechanical strength to the cross-linked polymeric gel network. These observations supported the successful crosslinking of PAAm polymeric chains with the alginate macromolecules in the given ratio.
O stretching vibrations, respectively, which confirms the crosslinking between the two groups. The presence of a peak at 1434 cm−1 in the Algi/PAAm beads corresponds to the C–N bending vibration, which further supports the crosslinking reaction. The sharp hydroxyl peak of alginate bead at 1620.90 cm−1 was observed to be split and shifted to a higher value of 1673.69 cm−1 and 1625.55 cm−1. The shift in the wavenumber of hydroxyl group of alginate bead may be due to an effect on bond formation involving adjacent hydroxyl groups in alginate and the CONH2 groups in PAAm as a result of the conformational changes in the crosslinked alginate and PAAm. A shift in the sharp peak at 649.07 cm−1 in PAAm to 612.51 cm−1 in Algi/PAAm bead was observed which could be attributed to NH2 bond wagging and bending vibration for the out of the plane carbon. The intermolecular hydrogen bonding provides an additional mechanical strength to the cross-linked polymeric gel network. These observations supported the successful crosslinking of PAAm polymeric chains with the alginate macromolecules in the given ratio.
Evaluation of hBMSCs encapsulated Algi/PAAm beads
Nevertheless, enhancing the ability of cells to elongate, migrate and connect with neighboring cells within 3D scaffold format is vital to recreate native tissue morphology and function in engineered tissues.24 Therefore, there is an urgent need for engineered tissues to mimic the native tissues, recreate the complex 3D cellular distribution and organization found in vivo, while maintaining the cell viability and function of the emulated tissues.25 In this study, morphologies of hBMSCs seeded onto 2D substrates were also compared to those cultured within the 3D bead matrix. While hBMSCs shows even spreading over the 2D surfaces, the cells encapsulated in the PAAm/alginate bead displays well-spread 3D interactions within the polymer network resulting into round shape morphology. 3D round morphology is the native cell morphology found in biological tissues.26,27 It is attributed as an advantage of the cell-laden bead matrix that can resemble the tissue-like microenvironments for their application in tissue engineering and regenerative medicine.28,29
4. Conclusion
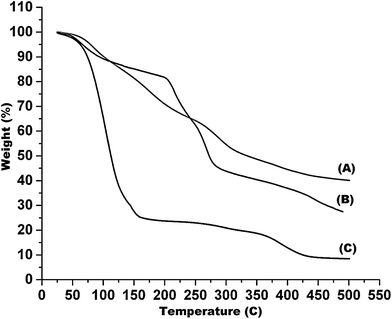
To best of our knowledge, this is the first report that demonstrates the development of cell-laden Algi/PAAm gels for hBMSCs delivery. Algi/PAAm gels were prepared and their physicochemical and biological properties were systematically characterized in the context of stem cell delivery. The in situ gelation approach has been followed for the encapsulation of hBMSCs within a selected ratio of the two polymers so as to minimize the detrimental effect of the polymers and their gelation mechanisms. The physicochemical properties of these quickly in situ gel-forming beads were investigated in terms of chemical group functionality (FTIR data), thermal degradation profiling (TGA and DSC data), internal porosity and interconnected pore morphology (SEM data). Stem cell encapsulation method within the bead matrix has been optimized and used to evaluate the cellular compatibility of the bead system using hBMSCs as model stem cells. The results confirmed the cellular viability as well as homogenous distribution of the cells within the bead matrix during the period of study (2 days). However, the reduced initial cell viability may be attributed to the encapsulation stress, nutrient limitations or stress due to transient swelling after placement in media. Additionally, it was observed that the hBMSCs cultured over 2D surfaces (culture plates) showed even spreading, whereas the cells encapsulated within the Algi/PAAm beads displayed well-spread 3D interactions within the polymer network resulting into round shape morphology. Other advantages of the bead system includes tunable system properties depending on cell type used as well as the application, easy preparation method (even possible in surgical theatre) and cost-effectiveness. The multi advantageous features make this gel bead system as an attractive stem cell carrier in general and hBMSCs in particular for stem cell delivery and scaffold-based tissue engineering application such as cartilage tissue regeneration. The results show that in situ forming beads from alginate and PAAm are suitable scaffolds to support the survival of hBMSCs with their potential in cartilage repair as well as their suitability as a novel stem cell delivery system.Conflict of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by CSCR. The author, Deepti Rana, would like to thank CSCR for the award of junior research fellowship.References
- S. Heilshorn, California's Stem Cell Agency, Grant no. RT2-01938, 2014, available from, https://www.cirm.ca.gov/our-progress/awards/preparation-and-delivery-clinically-relevant-numbers-stem-cells-using-3d.
- D. J. Mooney and H. Vandenburgh, Cell Stem Cell, 2008, 2, 205 CrossRef CAS PubMed
.
- D. E. Kim, K. Tsuji, Y. R. Kim, F. J. Mueller, H. S. Eom and E. Y. Snyder, et al., Radiology, 2006, 241, 822 CrossRef PubMed
.
- M. Kurdi, R. Chidiac, C. Hoemann, F. Zouein, C. Zgheib and G. W. Booz, Congestive Heart Failure, 2010, 16, 132 CrossRef PubMed
.
- C. Chai and K. W. Leong, Mol. Ther., 2007, 15, 467 CrossRef CAS PubMed
.
- C. C. Sheng, L. Zhou and J. Hao, BioMed Res. Int., 2013, 2013, 547902, DOI:10.1155/2013/547902
.
- X. Wei, X. Yang, Z. Han, F. Qu, L. Shao and Y. Shi, Acta Pharmacol. Sin., 2013, 34, 747 CrossRef CAS PubMed
.
- M. Ravi, V. Paramesh, S. R. Kaviya, E. Anuradha and F. D. Solomon, J. Cell. Physiol., 2015, 230, 16 CrossRef CAS PubMed
.
- M. Ramalingam and D. Rana, J. Bionanosci., 2015, 9, 13 CrossRef CAS
.
- H. Naito, M. Yoshimura, T. Mizuno, S. Takasawa, T. Tojo and S. Taniguchi, J. Biomed. Mater. Res., Part A, 2013, 101, 2838 CrossRef PubMed
.
- D. Rana, H. Zreiqat, N. Benkirane-Jessel, S. Ramakrishnan and M. Ramalingam, J. Tissue Eng. Regener. Med., 2015 DOI:10.1002/term.2061
.
- J. Sun and H. Tan, Materials, 2013, 6, 1285 CrossRef CAS
.
- L. Wang, R. R. Rao and J. P. Stegemann, Cells Tissues Organs, 2013, 197, 333 CrossRef CAS PubMed
.
- P. Guo, Y. Yuan and F. Chi, Mater. Sci. Eng., C, 2014, 42, 622 CrossRef CAS PubMed
.
- C. H. Yang, M. X. Wang, H. Haider, J. H. Yang, J.-Y. Sun and Y. M. Chen, et al., ACS Appl. Mater. Interfaces, 2013, 5, 10418 CAS
.
- L. H. Christensen, V. B. Breiting, A. Aasted, A. Jørgensen and I. Kebuladze, Plast. Reconstr. Surg., 2003, 111, 1883 CrossRef PubMed
.
- S. M. Burwell, Report on Carcinogens, NC, U.S, Research Triangle Park, NTP (National Toxicology Program), Department of Health and Human Services, Public Health Service, 13th edn, 2014 Search PubMed
.
- C. C. Doan, N. H. Truong, N. B. Vu, T. T. Nguyen, H. M. Nguyen and K. G. Nguyen, et al., Int. J. Plant, Anim. Environ. Sci., 2012, 2, 83 CAS
.
- L. Zou, Y. Luo, M. Chen, G. Wang, M. Ding and C. C. Petersen, et al., Sci. Rep., 2013, 3, 2243 Search PubMed
.
- A. F. A. Merrison, D. Gordon and N. J. Scolding, J. Cell Sci. Ther., 2012, 4, 001 CrossRef PubMed
.
- H.-L. Ma, S.-C. Hung, S.-Y. Lin, Y.-L. Chen and W.-H. Lo, J. Biomed. Mater. Res., Part A, 2003, 64, 273 CrossRef PubMed
.
- J.-Y. Sun, X. Zhao, W. R. K. Illeperuma, O. Chaudhuri, K. H. Oh and D. J. Mooney, et al., Nature, 2012, 489, 133 CrossRef CAS PubMed
.
- R. Tripathi and B. Mishra, AAPS PharmSciTech, 2012, 13, 1091 CrossRef CAS PubMed
.
- F. Tortelli and R. Cancedda, Eur. Cells Mater., 2009, 17, 1 CAS
.
- E. Carletti, A. Motta and C. Migliaresi, Methods Mol. Biol., 2011, 695, 17 CAS
.
- E. Knight and S. Przyborski, J. Anat., 2014, 227, 746 CrossRef PubMed
.
- M. P. Lutolf, P. M. Gilbert and H. M. Blau, Nature, 2009, 462, 433 CrossRef CAS PubMed
.
- M. W. Tibbitt and K. S. Anseth, Biotechnol. Bioeng., 2009, 103, 655 CrossRef CAS PubMed
.
- D. Rana, T. S. SampathKumar and M. Ramalingam, J. Biomater. Tissue Eng., 2014, 4, 507 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |