Microwave-assisted regioselective sulfenylation of indoles under solvent- and metal-free conditions†
Rajjakfur Rahaman,
Namita Devi,
Jyoti Rekha Bhagawati and
Pranjit Barman*
Department of Chemistry, National Institute of Technology Silchar, Silchar 788010, India. E-mail: barmanpranjit@yahoo.co.in; Fax: +91 3842 224797; Tel: +91 9435374128
First published on 9th February 2016
Abstract
Herein, we report a solvent- and metal-free methodology for the sulfenylation of indoles with sulfinic acids in the absence of an external catalyst under microwave irradiation. This environmentally friendly approach offered the desired products in moderate to excellent yields in only 10 min. Several functional group tolerances were monitored under the optimized conditions.
Introduction
Sulfenylated indole moieties are an important class of organosulfur compounds as they are present in many pharmaceutically and biologically important molecules.1 Among the sulfenylated indole derivatives, 3-sulfenylindoles have attracted considerable interest due to their greater therapeutic value in the treatment of several diseases (Chart 1), such as, HIV,2 heart disease,3 cancer,4 obesity5 and allergies.6 They are also used as potent inhibitors in tubulin polymerization.7 These fascinating biological profiles are the basic cause of long standing interest in the development of efficient methods for the synthesis of 3-sulfenylindoles. In the last few decades, a number of significant methods have been developed. Variety of sulfenylating reagents have been discovered as reaction partners during the synthetic efforts. For example, sulfenyl halides,8 disulfides,9 thiols,10 quinine mono-O,S-acetals,11 arylsulfonyl chlorides,12 N-thioimides,13 sulfonium salts,14 and sulfenyl hydrazides15 are the most effective thiolating reagents for the synthesis of 3-sulfenylindoles.Although several methodologies have been reported for the reaction of electrophilic sulfur species to indoles, but in most of the cases, they require long reaction times, harsh reaction conditions (such as stoichiometric strong base), toxic reagents, and use of large amount of metals and/or solvents. Thus, development of a new environmentally friendly synthetic method for the sulfenylation of indoles remains an important challenge in organic synthesis.
In organic transformations, such as, C–S and C–Se bond formations,16 the use of microwave irradiation can provide excellent yield in a very short reaction time.17 In addition, with the development of sustainable technologies, solvent-free conditions have emerged as a benign alternative for organic synthesis.18 In the existing green chemistry scenario, microwave assisted organic synthesis (MAOS) has attained the status of a new and fascinating discipline.19 Microwave irradiation and solvent-free microwave-assisted techniques have been used for the rapid synthesis of various compounds, which have received special attention in recent years.20 With the assistance of microwave irradiation, reactions can proceed faster to give higher yields, as compared to conventional methods.
Braga and co-workers21 reported a highly efficient and solvent-free method for the synthesis of 3-chalcogenyl-indoles. They have used disulfides as sulfenylating agents, DMSO as a stoichiometric oxidant, and molecular iodine (I2) as catalyst, under microwave irradiation.
Thiols and disulfides were used as sulfenylating agents in many cases, but they have some practical limitations. Thiols are toxic, volatile, and foul smelling, where as disulfides are expensive and moisture sensitive. In addition, disulfide needs to be prepared via oxidative coupling of thiols; an extra operational step which causes low atom economy.22
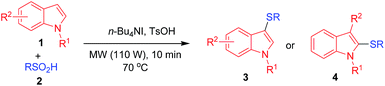
Recently, Liu et al. reported the sulfenylation of indoles, employing sulfinic acids as sulfenylating agents.23 Sulfinic acids are easily accessible, less costly, and are employed to form sulfones, which have versatile applications in medicinal chemistry.24 Moreover, sulfinic acids can also be reduced to disulfides, which are efficient sulfenylating agents. Taking this idea, herein, we report a fast and efficient method for the sulfenylation of indoles in the absence of solvent, under microwave irradiation (Scheme 1). The combination of a solvent-free reaction medium with microwave irradiation have been used successfully for the synthesis of 3-sulfenylation of indoles.25 However, to date, there are no reports of studies in which this attractive strategy has been applied to the synthesis of 3-sulfenylindoles. The protocol we have reported has a broad substrate scope, green reaction conditions, and high yields in a short reaction time.
Results and discussion
The reaction conditions were optimized for indole 1a and benzenesulfinic acid 2a taken as model substrates in the presence of tetrabutylammonium iodide (n-Bu4NI) and p-toluenesulfonic acid (Table 1). The reaction was carried out for reaction times of 3, 5, 7, and 10 min. The reaction for 10 min offered the desired product in highest yield (Table 1, entry 4).| Entry | 1a/2a (mmol) | MW (W) | T (°C) | Time (min) | Additive (mmol) | Acid (mmol) | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: indole 1a (0.5 mmol), benzenesulfinic acid 2a (0.6 mmol).b Isolated yield.c Conventional heating.d Reaction performed without microwave irradiation at room temperature. | |||||||
| 1 | 0.5/0.6 | 110 | 70 | 3 | n-Bu4NI (0.6) | TsOH (0.2) | 40 |
| 2 | 0.5/0.6 | 110 | 70 | 5 | n-Bu4NI (0.6) | TsOH (0.2) | 65 |
| 3 | 0.5/0.6 | 110 | 70 | 7 | n-Bu4NI (0.6) | TsOH (0.2) | 75 |
| 4 | 0.5/0.6 | 110 | 70 | 10 | n-Bu4NI (0.6) | TsOH (0.2) | 95 |
| 5 | 0.5/0.6 | 110 | 70 | 10 | n-Bu4NI (0.6) | TsOH (0.1) | 75 |
| 6 | 0.5/0.6 | 110 | 70 | 10 | n-Bu4NI (0.6) | TsOH (0.05) | 45 |
| 7 | 0.5/0.6 | 110 | 80 | 10 | n-Bu4NI (0.6) | TsOH (0.2) | 85 |
| 8 | 0.5/0.6 | 110 | 60 | 10 | n-Bu4NI (0.6) | TsOH (0.2) | 73 |
| 9 | 0.5/0.5 | 110 | 70 | 10 | n-Bu4NI (0.6) | TsOH (0.2) | 85 |
| 10 | 0.5/0.5 | 110 | 70 | 10 | n-Bu4NI (0.5) | TsOH (0.2) | 75 |
| 11 | 0.5/0.5 | 110 | 70 | 10 | n-Bu4NI (0.25) | TsOH (0.2) | 40 |
| 12 | 0.5/0.6 | 110 | 70 | 10 | n-Bu4NI (0.6) | HCl (0.2) | 70 |
| 13 | 0.5/0.6 | 110 | 70 | 10 | n-Bu4NI (0.6) | TfOH (0.2) | 85 |
| 14 | 0.5/0.6 | 110 | 70 | 10 | n-Bu4NI (0.6) | H2SO4 (0.2) | 45 |
| 15 | 0.5/0.6 | 150 | 70 | 10 | n-Bu4NI (0.6) | TsOH (0.2) | 86 |
| 16 | 0.5/0.6 | 70 | 70 | 10 | n-Bu4NI (0.6) | TsOH (0.2) | 64 |
| 17 | 0.5/0.6 | 110 | 70 | 10 | NaI (0.6) | TsOH (0.2) | 20 |
| 18 | 0.5/0.6 | 110 | 70 | 10 | KI (0.6) | TsOH (0.2) | 10 |
| 19 | 0.5/0.6 | 110 | 70 | 10 | — | TsOH (0.2) | 0 |
| 20 | 0.5/0.6 | — | 70 | 18 h | n-Bu4NI (0.6) | TsOH (0.2) | 80c |
| 21 | 0.5/0.6 | — | r.t. | 48 h | n-Bu4NI (0.6) | TsOH (0.2) | 76d |
In the next step, we examined the effect of temperature and influence of microwave irradiation in terms of yield. It was observed that at 70 °C and 110 W, the desired product 3a gives maximum yield of 97% (Table 1, entry 4).
Additionally, we examined the reaction under conventional heating in oil bath and at room temperature, which gives the desired product in 80% and 76% yield respectively. However, these processes required a very long reaction time (Table 1, entry 9 and 10).
After that, additive loading was screened to improve the yield of the product. On increasing the amount of additive (n-Bu4NI) to 0.6, 0.5, and 0.25 mmol; the desired product was obtained with 85, 75, and 40% yield (Table 1, entries 9–11). Thus, the additive loading was optimized at 0.6 mmol. However, there was no product formation without using n-Bu4NI (Table 1, entry 19). Other additives, such as NaI and KI were also evaluated, but were not as effective as n-Bu4NI (Table 1, entries 17, 18).
The effect of acids, such as, TsOH, HCl, TfOH, and H2SO4 were studied. It was observed that TsOH provided the desired product in highest yield, when used in stoichiometric amount. The best molar ratio of indole/sulfinic acid was found to be 0.5/0.6 (Table 1).
With the optimized reaction conditions in hand (Table 1, entry 4), the scope and limitations of the proposed method were investigated. First, we study the substrate scope of arylsulfinic acids towards indole (1a). A variety of arylsulfinic acids with electron donating and electron withdrawing groups were smoothly reacted with indole to form their corresponding 3-arylthioindoles, with moderate to excellent yields (Table 2). The arylsulfinic acids with electron donating groups, such as, –Me and –OMe on the phenyl ring produced the desired products with higher yields than those with electron withdrawing groups (–Cl, –Br, and –NO2). Thereafter, we have investigated the substrate scope of indoles. Similar trends were observed for indoles, where reactions with electron donating groups (–Me and –OMe) gives products with higher yields than those with electron withdrawing groups (–Br and –NO2). N-substituted indoles also offered the corresponding products with higher yields, without any difficulties. In general, sulfenylation occurs at 3-position of the indole ring (Table 2, 3a–3s). However, 2-position of the indole ring becomes the active reaction site, when 3-position is occupied by alkyl groups, such as, –Me (Table 2, 4a). No by-product (bis-2,3-arylthioindole) is obtained under the optimized reaction conditions.
To demonstrate the synthetic utility of the new method, gram scale reaction was carried out under the optimized conditions (Scheme 2). Thereby, the reaction between 1H-indole 1a and benzenesulfinic acid 2a, n-Bu4NI, and TsOH were taken in a 25 mL sealed glass vial and placed in the Milestone Srl microwave reactor. After, 45 min of reaction time the desired product 3a obtained in 80% yield.
In order to gain insight of the reaction mechanism, a control experiment was carried out with benzenesulfinic acid 2a, in the absence of indole 1a. This led to the formation of corresponding disulfide with excellent yield under optimized conditions (Scheme 3).23 During the reaction process, a purple colouration of the reaction mixture was observed, which confirmed the formation of iodine. In the presence of 20 mol% of iodine, indole 1a reacted with diphenyl disulfide 5a to give corresponding 3-sulfenylindole 3a in 95% yield. However, no product was formed on the treatment of diphenyl disulfide 5a with indole 1a, under the optimized reaction conditions.
On the basis of previous reports,9d,23,26 above experimental results, and control experiments, we proposed a plausible mechanism for 3-sulfenylation of indoles, as illustrated in Scheme 4. The stepwise removal of hydrogen and oxygen atoms from the SO2H group in sulfinic acid 2 in the presence of n-Bu4NI and TsOH, led to the formation of RSI. Alternatively, sulfenyl iodide reacts with 2 to give the sulfonothioate G. Reduction of G by n-Bu4NI/TsOH gives disulfide H.15 Disulfide H reacts with I2, which is produced in the earlier step to form sulfenyl iodide (F).9d,21 In the next step, indole 1 reacts with the sulfenyl iodide to form the intermediate I. I give the desired product 3. However, product 4 is formed when a substituent occupy the C-3 positions of indole 1. HI reacts with sulfinic acid and regenerate I2.
Conclusions
We have developed a fast, economic, and highly efficient MW-assisted synthetic method for the regioselective 3-sulfenylation of indoles using sulfinic acids as thiolating agent. The new approach gives the desired products in excellent yields in only 10 min, under metal- and solvent-free conditions. Important advantages associated with this methodology are: no side product is obtained on the completion of the reactions and the by-product I2 acts as an efficient catalyst. Due to excellent yield, short reaction time, and solvent-free conditions, this methodology promises to be a practical and greener alternative to earlier methods. This study will open a new window to many other useful transformations in organic synthesis. Further studies on the application of sulfinic acids are underway in our laboratory.Experimental
General methods and materials
All chemicals were purchased from commercially available sources and were used without further purification. Melting points were recorded on an electro thermal digital melting point apparatus and were uncorrected. 1H and 13C NMR spectra were recorded in CDCl3, DMSO-d6 and D2O at 600 MHz, 400 MHz, 150 MHz, 125 MHz, and 100 MHz. Chemical shifts (δ) are reported as parts per million (ppm) and are referenced to tetramethylsilane (TMS) as internal standard. NMR multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, m = multiplet, br = broad signal. Microwave-assisted syntheses were carried out in a monomode Milestone Srl microwave reactor. All reactions were performed in commercially available 10 mL sealed glass tubes. TLC was done on silica gel coated glass slide (Merck silica gel G for TLC). For column chromatography, silica gel 60–120 mesh (SRL, India) was used. Elemental analyses were performed on a Flash 2000 Thermo Scientific instrument. The yields are based on isolated compounds after purification.Typical procedure for the synthesis of 3-sulfenylindoles
Mixture of indole 1 (0.5 mmol), sulfinic acid 2 (0.6 mmol), tetrabutylammonium iodide (221.5 mg, 0.6 mmol), and TsOH (35.5 mg, 0.2 mmol) were taken in a sealed glass tube (10 mL) and placed in the microwave reactor. A maximum irradiation power of 110 W and 70 °C were applied for 10 min. When the temperature reached 70 °C, the instrument automatically adjust to maintain a constant temperature. After 10 min, the reaction mixture was cooled to room temperature. The reaction mixture was quenched with 10 mL aqueous solution of 10% sodium thiosulfate and the organic layer was extracted with ethyl acetate (3 × 10 mL). The organic layer was dried over anhydrous Na2SO4, filtered, and the solvent was removed under vacuum. The crude was purified by column chromatography on silica gel, with petroleum ether/ethyl acetate (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent, to get the desired product 3.
1) as the eluent, to get the desired product 3.
Acknowledgements
The authors are thankful to the Director, NIT Silchar for financial support. MHRD is also acknowledged for the doctorate fellowship received by R.F.R and N.D.Notes and references
- (a) R. L. Sundberg, Indoles, Academic, London, 1996 Search PubMed; (b) A. Casapullo, G. Bifulco, I. Bruno and R. Riccio, J. Nat. Prod., 2000, 63, 447 CrossRef CAS PubMed; (c) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed; (d) A. R. Katritzky and A. F. Pozharskii, Handbook of Heterocyclic Chemistry, Pergamon, Oxford, 2000 Search PubMed; (e) B. Bao, Q. Sun, X. Yao, J. Hong, C. O. Lee, C. J. Sim, K. S. Im and J. H. Jung, J. Nat. Prod., 2005, 68, 711 CrossRef CAS PubMed; (f) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (g) M. C. Van Zandt, M. L. Jones, D. E. Gunn, L. S. Geraci, J. H. Jones, D. R. Sawicki, J. Sredy, J. L. Jacot, A. T. Dicioccio, T. Petrova, A. Mischler and A. D. Podjarny, J. Med. Chem., 2005, 48, 3141 CrossRef CAS PubMed; (h) T. R. Garbe, M. Kobayashi, N. Shimizu, N. Takesue, M. Ozawa and H. Yukawa, J. Nat. Prod., 2000, 63, 596 CrossRef CAS PubMed; (i) G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045 RSC.
- R. Ragno, A. Coluccia, G. La Regina, G. De Martino, F. Piscitelli, A. Lavecchia, E. Novellino, A. Bergamini, C. Ciaprini, A. Sinistro, G. Maga, E. Crespan, M. Artico and R. Silvestri, J. Med. Chem., 2006, 49, 3172 CrossRef CAS PubMed.
- C. D. Funk, Nat. Rev. Drug Discovery, 2005, 4, 664 CrossRef CAS PubMed.
- G. La Regina, M. C. Edler, A. Brancale, S. Kandil, A. Coluccia, F. Piscitelli, E. Hamel, G. De Martino, R. Matesanz, J. F. Díaz, A. I. Scovassi, E. Prosperi, A. Lavecchia, E. Novellino, M. Artico and R. Silvestri, J. Med. Chem., 2007, 50, 2865 CrossRef CAS PubMed.
- (a) J. P. Berger, T. W. Doebber, M. Leibowitz, D. E. Moller, R. T. Mosley, R. L. Tolman, J. Ventre, B. B. Zhang and G. Zhou, PCT Int. Appl, WO 0130343, 2001transChem. Abstr., 2001, 134, 320871 Search PubMed; (b) V. S. N. Ramakrishna, V. S. Shirsath, R. S. Kambhampati, S. Vishwakarma, N. V. Kandikere, S. Kota and V. Jasti, PCT Int. Appl., WO 2007020653, 2007transChem. Abstr., 2007, 146, 274218 Search PubMed.
- P. C. Unangst, D. T. Connor, S. R. Stabler, R. J. Weikert, M. E. Carethers, J. A. Kennedy, D. O. Thueson, J. C. Chestnut, R. L. Adolphson and M. C. Conroy, J. Med. Chem., 1989, 32, 1360 CrossRef CAS PubMed.
- (a) G. De Martino, G. La Regina, A. Coluccia, M. C. Edler, M. C. Barbera, A. Brancale, E. Wilcox, E. Hamel, M. Artico and R. Silvestri, J. Med. Chem., 2004, 47, 6120 CrossRef CAS PubMed; (b) Y. Maeda, M. Koyabu, T. Nishimura and S. Uemura, J. Org. Chem., 2004, 69, 7688 CrossRef CAS PubMed.
- (a) P. Hamel, J. Org. Chem., 2002, 67, 2854 CrossRef CAS PubMed; (b) M. Raban and L.-J. Chern, J. Org. Chem., 1980, 45, 1688 CrossRef CAS.
- (a) L.-H. Zou, J. Reball, J. Mottweiler and C. Bolm, Chem. Commun., 2012, 48, 11307 RSC; (b) G. La Regina, V. Gatti, V. Famiglini, F. Piscitelli and R. Silvestri, ACS Comb. Sci., 2012, 14, 258 CrossRef CAS PubMed; (c) W. Ge and Y. Wei, Synthesis, 2012, 934 CAS; (d) W. Ge and Y. Wei, Green Chem., 2012, 14, 2066 RSC; (e) Z. Li, J. Hong and X. Zhou, Tetrahedron, 2011, 67, 3690 CrossRef CAS; (f) X.-L. Fang, R.-Y. Tang, P. Zhong and J.-H. Li, Synthesis, 2009, 4183 CAS; (g) C. C. Browder, M. O. Mitchell, R. L. Smith and G. el-Stdayman, Tetrahedron Lett., 1993, 34, 6245 CrossRef CAS; (h) P. Sang, Z. Chen, J. Zoua and Y. Zhang, Green Chem., 2013, 15, 2096 RSC; (i) Ch. D. Prasad, S. Kumar, M. Sattar, A. Adhikary and S. Kumar, Org. Biomol. Chem., 2013, 11, 8096 Search PubMed; (j) Y. Liu, H. Wang, C. Wang, J.-P. Wan and C. Wen, RSC Adv., 2013, 3, 21369 RSC; (k) R. Rahaman, N. Devi and P. Barman, Tetrahedron Lett., 2015, 56, 4224 CrossRef CAS.
- (a) J. S. Yadav, B. V. S. Reddy, Y. J. Reddy and K. Praneeth, Synthesis, 2009, 1520 CrossRef CAS; (b) J. S. Yadav, B. V. S. Reddy and Y. J. Reddy, Tetrahedron Lett., 2007, 48, 7034 CrossRef CAS; (c) Y. Maeda, M. Koyabu, T. Nishimura and S. Uemura, J. Org. Chem., 2004, 69, 7688 CrossRef CAS PubMed; (d) K. M. Schlosser, A. P. Krasutsky, H. W. Hamilton, J. E. Reed and K. Sexton, Org. Lett., 2004, 6, 819 CrossRef CAS PubMed; (e) J. A. Campbell, C. A. Broka, L. Gong, K. A. M. Walker and J.-H. Wang, Tetrahedron Lett., 2004, 45, 4073 CrossRef CAS; (f) Y. Liu, Y. Zhang, C. Hu, J.-P. Wana and C. Wen, RSC Adv., 2014, 4, 35528 RSC; (g) G. Wu, J. Wu, J. Wu and L. Wu, Synth. Commun., 2008, 38, 1036 CrossRef CAS; (h) Y. Liu, Y. Zhang, C. Hu, J.-P. Wan and C. Wen, RSC Adv., 2014, 4, 35528 RSC.
- (a) M. Matsugi, K. Murata, H. Nambu and Y. Kita, Tetrahedron Lett., 2001, 42, 1077 CrossRef CAS; (b) M. Matsugi, K. Murata, K. Gotanda, H. Nambu, G. Anilkumar, K. Matsumoto and Y. Kita, J. Org. Chem., 2001, 66, 2434 CrossRef CAS PubMed.
- Q. Wu, D. Zhao, X. Qin, J. Lan and J. You, Chem. Commun., 2011, 47, 9188 RSC.
- (a) E. Marcantoni, R. Cipolletti, L. Marsili, S. Menichetti, R. Properzi and C. Viglianisi, Eur. J. Org. Chem., 2013, 132 CrossRef CAS; (b) C. C. Silveira, S. R. Mendes, L. Wolf and G. M. Martins, Tetrahedron Lett., 2010, 51, 2014 CrossRef CAS; (c) M. Tudge, M. Tamiya, C. Savarin and G. R. Humphrey, Org. Lett., 2006, 8, 565 CrossRef CAS PubMed.
- S. Jain, K. Shukla, A. Mukhopadhyay, S. N. Suryawanshi and D. S. Bhakuni, Synth. Commun., 1990, 20, 1315 CrossRef CAS.
- F.-L. Yang and S.-K. Tian, Angew. Chem., Int. Ed., 2013, 52, 4929 CrossRef CAS PubMed.
- (a) Y. Ju, D. Kumar and R. S. Varma, J. Org. Chem., 2006, 71, 6697 CrossRef CAS PubMed; (b) G. V. Botteselle, M. Godoi, F. Z. Galetto, L. Bettanin, D. Singh, O. E. D. Rodrigues and A. L. Braga, J. Mol. Catal. A: Chem., 2012, 365, 186 CrossRef CAS; (c) J. B. Azeredo, M. Godoi, R. S. Schwab, G. V. Botteselle and A. L. Braga, Eur. J. Org. Chem., 2013, 5188 CrossRef CAS; (d) Q. Wu, D. Zhao, X. Qin, J. Lan and J. You, Chem. Commun., 2011, 47, 9188 RSC; (e) K. Görmer, H. Waldmann and G. Triola, J. Org. Chem., 2010, 75, 1811 CrossRef PubMed.
- (a) M. A. Herrero, J. M. Kremsner and C. O. Kappe, J. Org. Chem., 2008, 73, 36 CrossRef CAS PubMed; (b) M. Najeebullah, D. W. Knight, M. A. Munawar, A. Yaseenx and F. Vincenzo, Tetrahedron, 2010, 66, 6761 CrossRef CAS; (c) C. R. Strauss and D. W. Rooney, Green Chem., 2010, 12, 1340 RSC; (d) M. N. Nadagouda, T. F. Speth and R. S. Varma, Acc. Chem. Res., 2011, 44, 469 CrossRef CAS PubMed; (e) M. T. Barros, K. T. Petrova, P. Correia-da-Silva and T. M. Potewar, Green Chem., 2011, 13, 1897 RSC; (f) D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS PubMed; (g) A. L. Braga, M. W. Paixao, B. Westermann, P. H. Schneider and L. A. Wessjohann, J. Org. Chem., 2008, 73, 2879 CrossRef CAS PubMed; (h) P. Lidström, J. Tierney, B. Wathey and J. Westman, Tetrahedron, 2001, 57, 9225 CrossRef; (i) D. Obermayer, B. Gutmann and C. O. Kappe, Angew. Chem., Int. Ed., 2009, 48, 8321 CrossRef CAS PubMed; (j) C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250 CrossRef CAS PubMed.
- (a) R. S. Varma, Green Chem., 1999, 1, 43 RSC; (b) K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025 CrossRef CAS PubMed.
- (a) B. Gutmann, A. M. Schwan, B. Reichart, C. Gspan, F. Hofer and C. O. Kappe, Angew. Chem., Int. Ed., 2011, 50, 7636 CrossRef CAS PubMed; (b) J. D. Moseley and C. O. Kappe, Green Chem., 2011, 13, 794 RSC.
- (a) J. F. Collados, E. Toledano, D. Guijarro and M. Yus, J. Org. Chem., 2012, 77, 5744 CrossRef CAS PubMed; (b) A. J. A. Watson, A. C. Maxwell and J. M. J. Williams, J. Org. Chem., 2011, 76, 2328 CrossRef CAS PubMed; (c) J. A. Seijas, M. P. Vazquez-Tato and R. Carballido-Reboredo, J. Org. Chem., 2005, 70, 2855 CrossRef CAS PubMed; (d) S. Horikoshi, T. Hamamura, M. Kajitani, M. Yoshizawa-Fujita and N. Serpone, Org. Process Res. Dev., 2008, 12, 1089 CrossRef CAS.
- J. B. Azeredo, M. Godoi, G. M. Martins, C. C. Silveira and A. L. Braga, J. Org. Chem., 2014, 79, 4125 CrossRef CAS PubMed.
- (a) Y. Liu, H. Wang, C. Wang, J.-P. Wan and C. Wen, RSC Adv., 2013, 3, 21369 RSC; (b) H. Wang, G. Huang, Y. Sun and Y. Liu, J. Chem. Res., 2014, 96 CrossRef.
- C.-R. Liu and L.-H. Ding, Org. Biomol. Chem., 2015, 13, 2251 CAS.
- (a) C. J. Dinsmore, T. M. Williams, T. J. O'Neill, D. Liu, E. Rands, J. C. Culberson, R. B. Lobell, K. S. Koblan, N. E. Kohl, J. B. Gibbs, A. I. Oliff, S. L. Graham and G. D. Hartman, Bioorg. Med. Chem. Lett., 1999, 9, 3301 CrossRef CAS PubMed; (b) C.-R. Liu, M.-B. Li, D.-J. Cheng, C.-F. Yang and S.-K. Tian, Org. Lett., 2009, 11, 2543 CrossRef CAS PubMed; (c) Z.-Y. Sun, E. Botros, A.-D. Su, Y. Kim, E. Wang, N. Z. Baturay and C.-H. Kwon, J. Med. Chem., 2000, 43, 4160 CrossRef CAS PubMed; (d) C.-R. Liu and M. B. Li, Chin. J. Chem., 2013, 31, 1274 CrossRef CAS; (e) C.-R. Liu, F.-L. Yang and T.-T. Wang, Chin. J. Chem., 2014, 32, 387 CrossRef CAS; (f) T. Miao, P. Li, Y. Zhang and L. Wang, Org. Lett., 2015, 17, 832 CrossRef CAS PubMed; (g) Y. Xi, B. Dong, E. J. McClain, Q. Wang, T. L. Gregg, N. G. Akhmedov, J. L. Petersen and X. Shi, Angew. Chem., Int. Ed., 2014, 53, 4657 CrossRef CAS PubMed.
- (a) M. Godoi, E. W. Ricardo, G. V. Botteselle, F. Z. Galetto, J. B. Azeredo and A. L. Braga, Green Chem., 2012, 14, 456 RSC; (b) G. Perin, S. R. Mendes, M. S. Silva, E. J. Lenardao, R. G. Jacob and P. C. Santos, Synth. Commun., 2006, 36, 2587 CrossRef CAS; (c) G. Perin, R. G. Jacob, L. G. Dutra, F. Azambuja, G. F. F. Santos and E. J. Lenardao, Tetrahedron Lett., 2006, 47, 935 CrossRef CAS; (d) F. Xiao, H. Xie, S. Liu and G.-J. Deng, Adv. Synth. Catal., 2014, 356, 364 CrossRef CAS.
- (a) W. Ge and Y. Wei, Synthesis, 2012, 934 CAS; (b) Z. Li, J. Hong and X. Zhou, Tetrahedron, 2011, 67, 3690 CrossRef CAS; (c) X.-L. Fang, R.-Y. Tang, P. Zhong and J.-H. Li, Synthesis, 2009, 4183 CAS; (d) C. C. Browder, M. O. Mitchell, R. L. Smith and G. Stdayman, Tetrahedron Lett., 1993, 34, 6245 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR and 13C NMR spectra of synthesized compounds. See DOI: 10.1039/c5ra26425a |
| This journal is © The Royal Society of Chemistry 2016 |