A competitive fluorescence quenching-based immunoassay for bisphenol A employing functionalized silica nanoparticles and nanogold†
Wei Zhao,
Wei Ji,
Yuanfu Zhang,
Lingyun Du and
Shuhao Wang*
Shandong Provincial Key Laboratory of Chemical Energy Storage and Novel Cell Technology, School of Chemistry and Chemical Engineering, Liaocheng University, Liaocheng 252059, China. E-mail: shuhaowang@sohu.com; Fax: +86 635 8239227; Tel: +86 635 8239227
First published on 11th April 2016
Abstract
We have developed a fast and sensitive immunoassay for the determination of bisphenol A (BPA), using the fluorescence quenching effect between gold nanoparticles and fluorescein isothiocyanate (FITC). Herein, carboxyl-modified single-strand DNA (COOH-ssDNAs) and anti-BPA antibodies were simultaneously conjugated with silica nanoparticles, and FITC-labeled single-strand DNA (FITC-ssDNA, the complement of COOH-ssDNA) was hybridized with COOH-ssDNA to form a signal platform, and the BPA coated antigen-functionalized nanogold was regarded as an acceptor. In the presence of BPA, a competitive immunoreaction takes place between BPA and BPA coated antigen-functionalized nanogold for the binding sites of the anti-BPA antibodies on the signal platform. Due to the fact that the photoluminescence of FITC was strongly quenched by the AuNPs, the fluorescence emission was decreased significantly. Under optimized conditions, the fluorescence intensity had a linear signal response range with a BPA concentration from 2.0 × 10−3 ng mL−1 to 1.0 ng mL−1 with a low limit of detection of 1.44 × 10−3 ng mL−1. Furthermore, this new signal platform was successfully applied to detect real samples for low levels of BPA.
Introduction
With the development of industrial technology, BPA has been widely used in all walks of life. It is found in hundreds of plastic items from water bottles to CDs to dental sealants.1–3 However, incomplete manufacturing techniques and inappropriate use make it cause much potential harm. Because of its estrogenic activity, toxicity and bioaccumulation, these kinds of environmental endocrine disruptors interfere with the development of the reproductive and nervous systems and possibly promote cancer.4–6 Relevant data show that BPA is widely used in water bottles and food containers. In the process of eating, low doses of BPA material will be dissolved into solution under high temperature conditions. About 90 percent of Americans have traces of bisphenol A in their urine.7 While older children and adults eliminate the chemical through their kidneys, newborns and infants can retain it for much longer. So scientists pushing for a ban on the chemical argue that BPA mimics the effects of the hormone estrogen, interfering with growth. Therefore, monitoring of BPA requires a promising method to enable the screening of various samples.Over the decades, scientists dedicated to studying BPA seek more sensitive and convenient detection methods, and they have put forward many diverse ways including GC-MS,8 HPLC,9 CL-ELISA,10 RRS,11 fluorescence immunoassay (FIA),12 and so on. FIA using fluorescent labels and specificity of the antigen–antibody reaction provides high sensitivity and good selectivity.13,14 Fluorescent labels can provide tremendous sensitivity due to their discrete emission properties upon excitation. Nucleic acids and proteins can be labeled with fluorophores to make the determinand visible in assay processes, and keep their own specificity at the same time. Furthermore, in recent years huge developments have been made in combining biologicals with nano-particles.15,16 Nano-particles’ simple preparation method, size- or shape-controllable characteristics and biocompatibility promote their application in analytical chemistry. Among them, many great achievements have been made with gold nanoparticles (AuNPs) and silica nanoparticles (SiNPs).17,18 AuNPs are the most commonly used optical sensing component due to their high molar extinction coefficients and unique optical properties in the ultra violet-visible spectral region.19,20 They also possess a high surface-to-volume ratio and other properties related to their nanometer dimension. Silica nanoparticles used in bio-applications began with Stöber and co-workers in 1968.21 After that, silane modification techniques and silica derivatives passivated the nonspecific binding potential of raw silica. So silica nanoparticles present many excellent properties.22–25 Such as, their surface can be easily functionalized, then biologicals can be combined with them through covalent binding or physical absorption. In addition, unique photometric characteristics, nontoxicity, and good solubility in aqueous environments has led to silica nanoparticles being used in many applications. From the above, AuNPs and silica nanoparticles can offer suitable foundations for the fabrication of sensors in immunoassays.
Herein, an ultrasensitive immunoassay based on fluorescence quenching for the determination of BPA was set up. In the process of the whole experiment, silica nanoparticles were used as the foundation. Polyclonal rabbit antibodies of anti-BPA and carboxyl-modified single-strand DNA were integrated with silica nanoparticles via a condensation reaction, and the fluorescein isothiocyanate (FITC)-labeled single DNA was hybridized with COOH-ssDNAs to form the signal probes. Aside form that, the AuNPs coupled with the BPA coated antigens served as excellent fluorescence quenchers, and the assay depended on a competitive binding reaction between BPA and BPA coated antigen-functionalized nanogold for the binding sites of the anti-BPA antibodies on the signal probes.
Experimental
Materials and reagents
Polyclonal rabbit antibodies against BPA were obtained from our own laboratory. Modified oligonucleotide was synthesized and purified by Shanghai Sangon Biotechnology Co. The sequences of DNA are shown as follows.S1: 5′COOH – AGCTCTTCCCATACCGGTACCTGACACAG
S2: 5′FITC – TACTGCTGTGTCAGGTACCGGTATGGGAAGAG
N-Hydroxysuccinimide (NHS, ≥97.0%), N-hydroxysulfosuccinimide sodium salt (Sulfo-NHS, ≥98.5%), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC, commercial grade), N,N′-dicyclohexylcarbodiimide (DCC, ≥99.0%), MES (≥99.0%), 4,4-bis(4-hydroxyphenyl)valeric acid (BHPVA, 95%), bisphenol A (BPA, ≥99.0%), 4,4′-(1-phenylethylidene)bisphenol (BP-AP, 99.0%), 2,2-bis(4-hydroxy-3-methylphenyl)propane (BPC, 97.0%), bis(4-hydroxyphenyl)methane (BPF, 98.0%), 4,4′-sulfonyldiphenol (BPS, 98%), Coomassie brilliant blue G 250 (CBB G 250), were purchased from Sigma-Aldrich. Albumin bovine V (BSA), Tris–HCl, HAuCl4·3H2O were supplied by J&K Chemical Co. Amino-modified silica nanoparticles (∼70 nm) were produced by Germany Micromod. All other reagents were of analytical grade. Ultrapure water (≥18 MΩ cm) was obtained from Millipore water purification system.
Apparatus
In this study, the signal probes were separated from the suspensions using a Himac CR 22G high-speed refrigerated centrifuge (Hitachi Co., Japan). The representation of the signal probe properties were monitored using a UV-2550 UV-vis spectrometer (Shimadzu, Japan). All fluoroimmunoassay testing was measured using a Hitachi F-7000 spectrofluorimeter (Hitachi, Japan). The excitation wavelength was 490 nm, and the spectral region was between 495 and 590 nm.Preparation of coated antigens
The coated antigen was prepared using a method previously reported in the literature with slight modification.26 Briefly, 80 μmol BHPVA, 96 μmol NHS and 112 μmol DCC (molar ratio: 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) were added into 2 mL of DMF. Then the solution was stirred at room temperature overnight. Under vigorous stirring, the above mixture was dropped into 8 mL of 10.25 mg mL−1 BSA (sodium bicarbonate buffer, pH 7.0). The final solution dialyzed against phosphate buffer (pH 7.4) and H2O at 4 °C for 2 days. All steps were operated in the dark and the mixture was turbid during the whole processes. Finally, the dialysate was lyophilized and stored at −20 °C for use. According to the method of coomassie brilliant blue (CBB, G250) staining,27 the coupling ratio of BHPVA and BSA was 13.3
7) were added into 2 mL of DMF. Then the solution was stirred at room temperature overnight. Under vigorous stirring, the above mixture was dropped into 8 mL of 10.25 mg mL−1 BSA (sodium bicarbonate buffer, pH 7.0). The final solution dialyzed against phosphate buffer (pH 7.4) and H2O at 4 °C for 2 days. All steps were operated in the dark and the mixture was turbid during the whole processes. Finally, the dialysate was lyophilized and stored at −20 °C for use. According to the method of coomassie brilliant blue (CBB, G250) staining,27 the coupling ratio of BHPVA and BSA was 13.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Preparation of gold nanoparticles and coated antigen–AuNPs acceptor
The nanogold was synthesized according to the literature with slight modification.28 95 mL of ultrapure water and 1 mL of 1% (w/v) HAuCl4 were mixed and boiled. Then 4 mL of 1% (w/v) trisodium citrate was added rapidly into the boiling solution and heated for 15 minutes, a d colloidal gold were presented claret-red. The nanogold solution was cooled to room temperature and stored at 4 °C before use. In order to get the antigen–AuNPs, the AuNP solution was adjusted to a pH value of 9 with a 0.1 M K2CO3 solution, 20 μL 1.2 mg mL−1 BPA coated antigens were added into the 1.0 mL AuNP solution with gentle stirring at room temperature for 30 min. Then 2 M NaCl (dissolved in 10 mM PB buffer, pH 7.4) was added to the solution to a final concentration 10 mM and was allowed to stand for about 15 min. After that, the coupled coated antigen–AuNPs conjugate was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min, and dispersed in buffer solution (10 mM PB, pH 7.4) for use.
000 rpm for 20 min, and dispersed in buffer solution (10 mM PB, pH 7.4) for use.
Preparation of antibody–silicon ball–DNA signal probes
Before combining with silicon, the antibodies and COOH-DNA were activated first. 15 μL 0.5 mg mL−1 of antibodies and 5 μL of 100 μM COOH-DNA were added into the activation buffer29 (100 mM MES, 5 mM NaCl, 2 mM EDC, 5 mM Sulfo-NHS, pH 6.0) for 30 minutes at room temperature. Then the activated IgG and COOH-DNA were separated from the excess agent by dialysis in MES and water. Afterwards, the activated solution was adjusted to a pH value of 7.4 by adding 2 M of sodium bicarbonate before use.In this study, the diameter of the silicon balls used as biological carriers is about 70 nm and their concentration is 25 mg mL−1. The silicon balls were washed three times with 100 mM MES (pH 6.0) before use. After slowly shaking, 20 μL of the silicon ball aqueous solution was added into 1 mL activated solution. The solution was mixed well and reacted for 2 hours at room temperature. It then underwent refrigerated centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm three times, the supernatant was removed and the opalescent sediment was dispersed in blocking buffer (10 mM PB, 5 mM NaCl, 0.15% BSA, pH 7.4). The solution was centrifuged again and the sediment was suspended in buffer (10 mM PB, 5 mM NaCl, pH 7.4). Afterward, 5 μL of the FITC labeled ssDNA was added and incubated at room temperature for some time. It was then centrifuged, and the silica nanoparticle signal probes were resuspended and stored at 4 °C until use.
000 rpm three times, the supernatant was removed and the opalescent sediment was dispersed in blocking buffer (10 mM PB, 5 mM NaCl, 0.15% BSA, pH 7.4). The solution was centrifuged again and the sediment was suspended in buffer (10 mM PB, 5 mM NaCl, pH 7.4). Afterward, 5 μL of the FITC labeled ssDNA was added and incubated at room temperature for some time. It was then centrifuged, and the silica nanoparticle signal probes were resuspended and stored at 4 °C until use.
Competitive fluorescence immunoassay
250 μL of BPA standard solution or BPA sample and 250 μL of 2.4 nM coated antigen–AuNPs were pipetted into a centrifuge tube, then 500 μL functionalized silica nanoparticles were added into the mixed solution. It was incubated at 37 °C for 40 min with strong shaking. Finally, the fluorescence intensity of the incubated solution was measured from 495 nm to 590 nm with an excitation wavelength of 490 nm and then the standard curve was drawn for use.Sample collection and preparation
The samples, consisting of food cartons (e.g., fast-food wrapped), printing papers (e.g., A4 printing papers), and Dixie cups were collected from supermarkets in China. The packaging materials were cut up and the fragments (0.1 g) were immersed in 100 mL of 65% (w/v) ethanol aqueous solution. Then the mixture was heated at 80 °C for 6 h. The final solution was concentrated by rotary evaporation, and dissolved in 20% (w/v) ethanol aqueous solution.Results and discussion
Principle of the immunoassay based on fluorescence quenching
In this work, an immunoassay was designed using functionalized silica nanoparticles and nanogold. Scheme 1 depicts the analytical process for BPA. SiNPs and AuNPs all have ultrahigh surface areas for loading multiple molecules as the carriers. In the process, anti-BPA antibodies and FITC-labeled DNA were conjugated with silica nanoparticles as the donor. Additionally, AuNPs were functionalized with BPA coated antigens as acceptors. Due to AuNPs having high molar extinction coefficients, this makes the corresponding fluorescence decrease or quench. In the whole experiment, BPA competes with the coated antigen–AuNPs to bind the antibody–silicon ball–DNA. With an increase in the content of BPA, there is a small amount of the coated antigen–AuNPs combined with the FITC-labeled SiNP donors, and the quenching response is weakened. Then the concentration of BPA is measured by the increase of fluorescence intensity.Characteristics of the coated antigen–AuNPs and antibodies–silicon ball–DNA
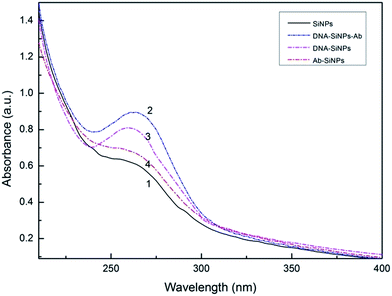
The synthesized nanogold solution was dispersed uniformly, and the average diameter of the AuNPs was about 13 nm. The spectral analysis of the colloidal gold revealed that a characteristic absorption peak appears at 519 nm. According to the Lambert–Beer law, the concentration of colloidal gold was about 3.7 nM (ε520 = 2.7 × 108 M−1 cm−1).30 After the coated antigen was coupled with the gold nanoparticles to form acceptors, the absorption peak became red shifted at 523 nm (Fig. S1†). The results indicated that the particle size of the coated antigen–AuNPs was larger than that of the Au-NPs, which roughly revealed that the coated antigen was combined with Au-NPs together.31 Secondly, the coated antigen–AuNPs were also confirmed by TEM (Fig. S2†). The final concentration of the well-dispersed coated antigen–AuNPs was about 2.4 nM.The absorption spectrum of the silica nanoparticles and the conjugate were studied (Fig. 1). The results indicated that silica nanoparticles themselves in the UV-vis region have no obvious absorption peak. After coupling with DNA and antibodies, the compound has an observable characteristic absorption peak at 260 nm. The dual-codified silica nanoparticles were also confirmed using SEM (Fig. S3†).
In order to prove the feasibility of the experiment, the fluorescence spectra of the detection system were detected with different concentrations of coated antigen–AuNPs and pure nanogold balls into a functionalized silicon ball solution. The results illustrated that functionalized silicon balls have a strong fluorescent signal and gold has a strong fluorescence quenching effect to FITC with different concentrations of coated antigen–AuNPs from 0.68 nM to 2.4 nM (Fig. 2). So, with an increase of the functionalized gold concentrations, fluorescence intensity is gradually diminished.
Optimization of the preparation conditions for coated antigen–AuNPs and antibodies–silicon ball–DNA
The optimal concentration of coated antigen (BHPVA–BSA) for the preparation of coated antigen–AuNPs is measured using a colorimetric method. The final content corresponds to the minimum concentration which keeps the AuNP solution stable, appearing pink and without any coagulation phenomenon. Fig. S4† shows the strength of the characteristic absorption peak at 580 nm, which represents the coagulation of colloidal gold and gold becoming large particles. Under the conditions of high salinity, protein can increase the stability of the colloidal gold. So 20.0 μL of 1.2 mg mL−1 coated antigen was chosen as the optimized amount.In addition, the concentrations of anti-BPA antibody and SH-ssDNA were determined for the preparation of functionalized SiNPs. Firstly, the effect of the concentration of the anti-BPA antibody was examined. With a high antibody concentration, the linear range of the detection would be wide, but the sensitivity would be low. On the contrary, with a low antibody concentration, the linear range of detection would be low, and the sensitivity would be high. Considering all these factors, 15 μL of 0.5 mg mL−1 anti-BPA antibody was chosen as the optimum amount. Under the 0.5 mg mL−1 anti-BPA antibody, the effect of the concentration of DNA was examined. In Fig. S5,† with an increase in DNA, the fluorescence quenching efficiency steadily rises. In the presence of 5 μL of 100 μM ssDNA, the fluorescence quenching efficiency reaches a peak and then falls quickly. This behaviour occurs because a concentration of DNA that is too low corresponds to a weak fluorescence signal, but a concentration of DNA that is too high could suppress the combination of antibodies and silicone balls, thereby reducing the fluorescence quenching efficiency.
Optimization of detection conditions
The performance of the developed BPA assay is influenced by the assay conditions, such as its acidity and alkalinity values, and its salinity. Different assay conditions were investigated in our studies.Sensitivity and selectivity
Under optimal conditions, all measurements were conducted at room temperature. As seen from Fig. 3, the difference in fluorescence intensity is exponential based on the concentration of BPA. However, the calibration plots show a good linearity. The linear regression equation is F = 727.5 + 193.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) lg
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CBPA (ng mL−1) in the range from 2.0 × 10−3 ng mL−1 to 1.0 ng mL−1 with R = 0.9940. The limit of detection (LOD) calculated using three times the standard deviation of blanks was 1.44 × 10−3 ng mL−1.
CBPA (ng mL−1) in the range from 2.0 × 10−3 ng mL−1 to 1.0 ng mL−1 with R = 0.9940. The limit of detection (LOD) calculated using three times the standard deviation of blanks was 1.44 × 10−3 ng mL−1.
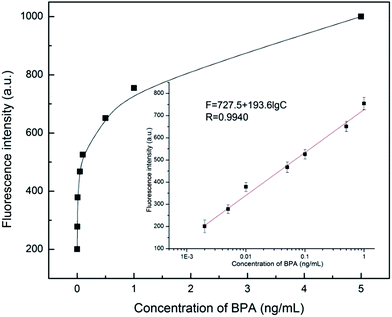 | ||
| Fig. 3 The fluorescence intensity changed with the variation of BPA concentration. Inset: magnification of the plot in the range of 2.0 × 10−3 ng mL−1 to 1.0 ng mL−1. | ||
BP-AP, BPC, BPF, BPS and DES were used as interferents to investigate the specificity of the immunoassay. The corresponding fluorescence intensity for each analogue is presented in Fig. 4. The results revealed that only BPA could significantly induce the change of fluorescence, and other analogue components generated weak fluorescence intensity. So this new immunoassay based on fluorescence quenching was shown to be sufficient for the qualitative diagnosis of BPA.
 | ||
| Fig. 4 The selectivity of the assay against BPA or a BPA congener. BPA, 5.00 × 10−3 ng mL−1, other congener, 5.00 × 10−3 ng mL−1 (n = 6). | ||
Analysis of real samples
To monitor the analytical reliability and applicable potential of this immunoassay for testing real samples, we collected 3 kinds of packages with various BPA levels from supermarket. These packaging materials are all thermoplastic materials. BPA in this packaging is not chemically bound but is bound by van der Waals forces. As seen from Table 1, through the conversion, a concentration of BPA between 2.9 ng g−1 to 6.7 ng g−1 is found in all kinds of packages. In order to guarantee the accuracy of the experiment, standard addition recovery tests were performed. The satisfactory results are also listed in Table 1.| Samples | Added (pg mL−1) | Found (pg mL−1) | Recovery (%) |
|---|---|---|---|
| Food cartons | 0.00 | 2.90 ± 0.21 | — |
| 2.00 | 5.03 ± 0.38 | 106.5 | |
| 4.00 | 6.84 ± 0.19 | 98.5 | |
| Printing papers | 0.00 | 5.31 ± 0.32 | — |
| 2.00 | 7.34 ± 0.27 | 101.5 | |
| 4.00 | 9.55 ± 0.23 | 106.0 | |
| Dixie cups | 0.00 | 6.73 ± 0.29 | — |
| 2.00 | 8.59 ± 0.35 | 93.0 | |
| 4.00 | 10.30 ± 0.44 | 89.2 |
Conclusions
In this work, based on a competing reaction a novel highly sensitive immunoassay for the detection of BPA was developed. This method used the fluorescence quenching effect and biological affinity of AuNPs skilfully. In addition to that, the use of silica nanoparticles made the reaction happen in a homogeneous solution, avoiding the process of repeated washing steps and being more convenient to separate than traditional methods. Otherwise, compared with traditional fluorescence immunoassays, silica nanoparticles provide a larger surface area than planar carriers for DNA and antibodies to combine, which can enhance the fluorescence intensity and improve the sensitivity of this method to some extent. We have used this method to test real samples from our daily life and have obtained a satisfactory result. So this novel immunoassay holds great promise as a sensitive, efficient and labour-saving strategy for BPA detection.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 20877037) and the Natural Science Foundation of Shandong Province, China (No. ZR2015BL030).References
- C. A. Staples, P. B. Dome, G. M. Klecka, S. T. Oblock and L. R. Harris, A review of the environmental fate, effects, and exposures of bisphenol A, Chemosphere, 1998, 36, 2149–2173 CrossRef CAS PubMed.
- L. N. Vandenberg, R. Hauser, M. Marcus, N. Olea and W. V. Welshons, Human exposure to bisphenol A (BPA), Reprod. Toxicol., 2007, 24, 139–177 CrossRef CAS PubMed.
- Y. Lu, J. R. Peterson, J. J. Gooding and N. A. Lee, Development of sensitive direct and indirect enzyme-linked immunosorbent assays (ELISAs) for monitoring bisphenol-A in canned foods and beverages, Anal. Bioanal. Chem., 2012, 403, 1607–1618 CrossRef CAS PubMed.
- K. L. Howdeshell, A. K. Hotchkiss, K. A. Thayer, J. G. Vandenbergh and F. S. VomSaal, Environmental toxins: exposure to bisphenol A advances puberty, Nature, 1999, 401, 763–764 CrossRef CAS PubMed.
- J. Hu, T. Aizawa and S. Ookubo, Products of aqueous chlorination of bisphenol A and their estrogenic activity, Environ. Sci. Technol., 2002, 36, 1980–1987 CrossRef CAS PubMed.
- S. Takayanagi, T. Tokunaga, X. Liu, X. H. Liu, H. Okada, A. Matsushima and Y. Shimohigashi, Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity, Toxicol. Lett., 2006, 167, 95–105 CrossRef CAS PubMed.
- M. K. Morgan, P. A. Jones, A. M. Calafat, X. Y. Ye, C. W. Croghan, J. C. Chuang, N. K. Wilson, M. S. Clifton, Z. Figueroa and L. S. Sheldon, Assessing the quantitative relationships between preschool children’s exposures to bisphenol A by route and urinary biomonitoring, Environ. Sci. Technol., 2011, 45, 5309–5316 CrossRef CAS PubMed.
- C. Nicolucci, S. Rossi, C. Menale, E. M. del Giudice, L. Perrone, P. Gallo, D. G. Mita and N. Diano, A high selective and sensitive liquid chromatography-tandem mass spectrometry method for quantization of BPA urinary levels in children, Anal. Bioanal. Chem., 2013, 405, 9139–9148 CrossRef CAS PubMed.
- X. Zhou, J. P. Kramer, A. M. Calafat and X. Y. Ye, Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 944, 152–156 CrossRef CAS PubMed.
- E. Maiolini, E. Ferri, A. L. Pitasi, A. Montoya, M. D. Giovanni, E. Errani and S. Girotti, Bisphenol A determination in baby bottles by chemiluminescence enzyme-linked immunosorbent assay, lateral flow immunoassay and liquid chromatography tandem mass spectrometry, Analyst, 2014, 139, 318–324 RSC.
- D. M. Yao, G. Q. Wen and Z. L. Jiang, A highly sensitive and selective resonance Rayleigh scattering method for bisphenol A detection based on the aptamer-nanogold catalysis of the HAuCl4–vitamin C particle reaction, RSC Adv., 2013, 3, 13353–13356 RSC.
- J. Zhang, S. Q. Zhao, K. Zhang and J. Q. Zhou, Cd-doped ZnO quantum dots-based immunoassay for the quantitative determination of bisphenol A, Chemosphere, 2014, 95, 105–110 CrossRef CAS PubMed.
- R. A. Houghten, General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen–antibody interaction at the level of individual amino acids, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 5131–5135 CrossRef CAS.
- M. Hall, I. Kazakova and Y. M. Yao, High sensitivity immunoassays using particulate fluorescent labels, Anal. Biochem., 1999, 272, 165–170 CrossRef CAS PubMed.
- M. C. Daniel and D. Astruc, Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- M. C. Hersam, N. P. Guisinger and J. W. Lyding, Silicon-based molecular nanotechnology, Nanotechnology, 2000, 11, 70 CrossRef CAS.
- C. S. Thaxton, D. G. Georganopoulou and C. A. Mirkin, Gold nanoparticle probes for the detection of nucleic acid targets, Clin. Chim. Acta, 2006, 363, 120–126 CrossRef CAS PubMed.
- J. Zhang, B. Liu, H. Liu, X. B. Zhang and W. H. Tan, Aptamer-conjugated gold nanoparticles for bioanalysis, Nanomedicine, 2013, 8, 983–993 CrossRef CAS PubMed.
- M. Horisberger, Colloidal gold: a cytochemical marker for light and fluorescent microscopy and for transmission and scanning electron microscopy, Scanning Electron Microsc., 1980, 2, 9–31 Search PubMed.
- P. Alexandridis, Gold nanoparticle synthesis, morphology control, and stabilization facilitated by functional polymers, Chem. Eng. Technol., 2011, 34, 15–28 CrossRef CAS.
- W. Stöber, A. Fink and E. Bohn, Controlled growth of monodisperse silica spheres in the micron size range, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- L. Wang, K. Wang, S. Santra, X. J. Zhao, L. R. Hilliard, J. E. Smith, Y. R. Wu and W. H. Tan, Watching silica nanoparticles glow in the biological world, Anal. Chem., 2006, 78, 646–654 CrossRef.
- S. H. Wu, Y. Hung and C. Y. Mou, Mesoporous silica nanoparticles as nanocarriers, Chem. Commun., 2011, 47, 9972–9985 RSC.
- Z. Li, J. C. Barnes, A. Bosoy, J. F. Stoddart and J. Zink, Mesoporous silica nanoparticles in biomedical applications, Chem. Soc. Rev., 2012, 41, 2590–2605 RSC.
- P. L. Kole, G. Venkatesh, J. Kotecha and R. Sheshala, Recent advances in sample preparation techniques for effective bioanalytical methods, Biomed. Chromatogr., 2011, 25, 199–217 CrossRef CAS PubMed.
- Y. Feng, Y. Zhou, Q. Zou, J. Wang, F. Chen and Z. Gao, Preparation and characterization of bisphenol A-cationized bovine serum albumin, J. Immunol. Methods, 2009, 340, 138–143 CrossRef CAS PubMed.
- J. J. Sedmak and S. E. Grossberg, A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250, Anal. Biochem., 1977, 79, 544–552 CrossRef CAS PubMed.
- G. Frens, Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions, Nature, 1973, 241, 20–22 CAS.
- R. Timkovich, Detection of the stable addition of carbodiimide to proteins, Anal. Biochem., 1977, 79, 135–143 CrossRef CAS PubMed.
- R. Jin, G. Wu, Z. Li, C. A. Mirkin and G. C. Schatz, What controls the melting properties of DNA-linked gold nanoparticle assemblies?, J. Am. Chem. Soc., 2003, 125, 1643–1654 CrossRef CAS PubMed.
- F. Y. Qiao, J. Liu, F. R. Li, X. L. Kong, H. L. Zhang and H. X. Zhou, Antibody and DNA dual-labeled gold nanoparticles: stability and reactivity, Appl. Surf. Sci., 2008, 254(10), 2941–2946 CrossRef CAS.
- A. Sinz, Chemical cross-linking and mass spectrometry for mapping three-dimensional structures of proteins and protein complexes, J. Mass Spectrom., 2003, 38, 1225–1237 CrossRef CAS PubMed.
- Z. Grabarek and J. Gergely, Zero-length crosslinking procedure with the use of active esters, Anal. Biochem., 1990, 185, 131–135 CrossRef CAS PubMed.
- S. G. Penn, L. He and M. J. Natan, Nanoparticles for bioanalysis, Curr. Opin. Chem. Biol., 2003, 7, 609–615 CrossRef CAS PubMed.
- N. Rohland, DNA extraction of ancient animal hard tissue samples via adsorption to silica particles, Methods Mol. Biol., 2012, 840, 21 Search PubMed.
- G. T. Hermanson, Bioconjugate techniques, Academic press, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26366b |
| This journal is © The Royal Society of Chemistry 2016 |