Physiochemical, optical and mechanical properties of poly(lactic acid) nanocomposites filled with toluene diisocyanate grafted cellulose nanocrystals
Jae-Gyoung Gwona,
Hye-Jung Choa,
Sang-Jin Chuna,
Soo Leeb,
Qinglin Wuc and
Sun-Young Lee*a
aNational Institute of Forest Science, Department of Forest Products, Seoul, 02455, Korea. E-mail: nararawood@korea.kr
bChangwon National University, Department of Chemical Engineering, Changwon, 51140, Korea
cLouisiana State University Agricultural Center, School of Renewable Natural Resources, Baton Rouge, LA 70803, USA
First published on 14th January 2016
Abstract
Although cellulose nanocrystals (CNCs) have been highlighted as a potential nano-reinforcement in polymer composites, the hydrophilic surface nature of CNCs limits their usage in the composite area due to the poor dispersibility of the CNCs in nonpolar solvent systems. A chemical modification of the CNCs was performed using toluene diisocyanate (TDI) to overcome the limitation in the solvent systems. CNCs and TDI-modified CNCs (mCNCs) reinforced poly(lactic) acid (PLA) films were prepared using the solvent casting method. After the modification, the mCNCs were well dispersed in chloroform with hydrophobic nature. In addition, AFM images and UV-Vis spectroscopy provide clear evidence for a good distribution of mCNCs in the PLA matrix. Thermal and mechanical behaviors of the PLA nanocomposite films with the CNCs and mCNCs were explored based on interfacial forces existing between the surface of the CNCs and the PLA matrix as well as the dispersibility of the CNCs with the increase of their loading levels.
1. Introduction
Cellulose nanocrystals (CNCs) as one of the nanocelluloses have the dimensions of 5–70 nm diameter and 100 nm (from plant fibers) to several micrometers (from cellulose of tunicates, algae, bacteria) length, and are usually prepared from the acid hydrolyzed amorphous region in cellulose fibers.1,2 Nanocelluloses as promising materials in high-tech material fields have a wide range of applications such as electronic devices,3,4 membranes for water treatment,5 oil absorbers,6 paper films,7 and polymer nanocomposites.8,9 Particularly, studies on the CNCs as a biodegradable nano-reinforcement have gained popularity in polymer nanocomposite fields because the materials applied with the CNCs show remarkable mechanical and physicochemical properties overcoming the limitations of composites incorporated with micro-scale reinforcement. Also, their ubiquity and sustainable productivity in nature have inspired us to study bio-nanocomposite alternatives to petroleum-based composites for fulfillment of environmental regulations.The development of biodegradable films with high performance in their mechanical properties is one of the most important challenges of the packaging industry today due to eco-friendly decomposition of the films in a sustainable manner.10 Poly(lactic acid) (PLA) as a commercially available biopolymer has been highlighted as a good candidate for packaging materials because of its biodegradability. Moreover, PLA is one of the few biopolymers available today which have similar properties to the petroleum-based commodity thermoplastics.11 Therefore, it can be expected to notably fortify the properties of the PLA films reinforced with the nanocelluloses such as biodegradability and biocompatibility, high stiffness, and low density compared to pure PLA film,12 and this enhancement in the properties makes PLA films have extensive applications.
However, there are some limitations in preparation of PLA films reinforced the nanocelluloses. The PLA film is mostly prepared by organic solvent (chloroform) casting method, but the nanocelluloses have strong hydrophilic nature due to abundant hydroxyl groups on its surface (three hydroxyl groups per glucose unit). Hence, a good dispersed suspension of the nanocelluloses in the nonpolar solvent is not easy to obtain. The poor dispersion in the solvent system can lead severe aggregation of the nanoparticles. Although the hydrophilicity of the nanocelluloses is possible to act as a good nano-biofiller in an aqueous polymer suspension,13 its surface characteristic limits their usage as nano-reinforcement for the composite processes in hydrophobic organic solvent systems. Therefore, it is essential to tune the surface of the nanocelluloses for overcoming the drawbacks representing in the organic solvent systems.
Recently extensive studies have been carried out on techniques for modulating the surface characteristic of the nanocelluloses. Chemical modification of the nanocellulose is generally achieved via the formation of covalent bond, and the modification is categorized into five strategies excepted for the pre-treatment methods: (i) urethanization,14–17 which forms urethane linkage by reaction with isocyanate group; (ii) silylation,12,18–22 which introduce silanol groups onto the surface through forming ether (–O–) or urethane (–NHCOO–) linkage; (iii) amidation,23 which forms amide linkage (–NHCO–) by the reaction of carboxylated site with amine group; (iv) acetylation,24 which forms ester linkage (–COO–) by reaction with acetic anhydride; (v) polymer grafting (‘onto’ or ‘from’).25–27
Urethanization leading an addition reaction causes no by-products, which require elimination during the modification like silylation leading condensation reaction. Also, isocyanate functionality (–NCO) has high reaction activity, and hence can react with various functionalities such as amino (–NH2), hydroxyl (–OH), and carboxylic acid (–COOH) groups. The high reactivity of the isocyanate group can render its chemistry to complicate, but it is possible to design various experimental approaches for desired chemical reactions. Despite above interesting advantages of urethanization, the surface tuning of the nanocellulose through urethanization strategy has received little attention compared to other surface tuning methods.
In the present work, our aims are to modify surface of cellulose nanocrystal (CNC) by using urethanization technique and to successfully disperse CNCs in PLA matrix for PLA nanocomposites. Toluene diisocyanate (TDI) was used for improving dispersibility of the CNCs in nonpolar organic solvents and compatibility of the CNCs with PLA. Particularly, TDI can lead many interactions with PLA compared with polyolefin such as polypropylene and polyethylene. The toluene moiety in TDI can interact with methyl group in PLA chain, and also its isocyanate group can react with hydroxyl group and carboxyl group at the chain ends (more details discussed later). Therefore, it can be expected that a continuous phase can be formed between PLA matrix and CNCs through TDI modification of CNCs. To the best our knowledge, TDI has not been reported in the surface modification area of the nanocellulose. Effects of the TDI on the dispersibility and the compatibility in the preparation of the PLA nanocomposites films were explored with various angles such as chemical, mechanical and optical properties. Meanwhile, a solvent exchange technique was adapted to obtain never-dried CNCs having no strong hydrogen bonds between the nanoparticles during the CNC manufacturing process.
2. Experimental
2.1. Materials
Cellulose powder (W-50, average particle size ∼45 μm, KC Flock) used as source of cellulose nanocrystals (CNCs) was purchased from Nippon Paper Chemicals Co., Ltd. Amorphous poly(lactic acid) (grade: 4060D with a L-lactide content of 88 wt%,28 Mw = 87 kDa29) was supplied by Nature Works. Toluene diisocyanate (TDI, 2,4-TDI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,6-TDI = 80
2,6-TDI = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, Alfa Aesar) was adapted for chemical modification of the CNCs. Sulfuric acid for acid hydrolysis of the cellulose powder was obtained from Junsei Chemical Co., Ltd. and was diluted to 64 wt% prior to use. Deionized water (DI-water, resistivity > 18 MΩ) was used for acid hydrolysis. Acetone (Ac), N,N-dimethylformamide (DMF), dichloromethane (DCM), and chloroform (CF) (HPLC grade, from Daejung Chemicals Co., Ltd.) were used for the solvent exchange as received.
20, Alfa Aesar) was adapted for chemical modification of the CNCs. Sulfuric acid for acid hydrolysis of the cellulose powder was obtained from Junsei Chemical Co., Ltd. and was diluted to 64 wt% prior to use. Deionized water (DI-water, resistivity > 18 MΩ) was used for acid hydrolysis. Acetone (Ac), N,N-dimethylformamide (DMF), dichloromethane (DCM), and chloroform (CF) (HPLC grade, from Daejung Chemicals Co., Ltd.) were used for the solvent exchange as received.
2.2. Preparation of cellulose nanocrystals
CNCs were extracted from a commercial cellulose powder by sulfuric acid hydrolysis. The procedure regarding the disintegration is drawn in Scheme 1a. Oven dried cellulose powders (at 80 °C for 24 h, 21 g) were hydrolyzed by 64 wt% sulfuric acid (350 ml) at 45 °C for 45 min with mechanical stirring. After the hydrolysis, the suspension was quickly poured into 2500 ml DI-water in order to stop the reaction. The supernatant was eliminated after 12 h, and then fresh DI-water was poured into the remained sediment again. This decanting step was repeated twice for 24 h. After removing the supernatant, the sediment was collected and then was neutralized by adding 10 M NaOH solution. Charged sulfate groups onto the sulfuric acid hydrolyzed CNCs enable the stable dispersion of CNCs in polar solvents because of electrostatic repulsion.30 In the following solvent exchange step, the formation of stable suspension can be hard to separate CNCs from solvents. The difficulty in separation between solvents and CNCs leads too much loss of CNCs as removed solvent. Hence, in this study, centrifugation of neutralized CNC suspension with rich ions for solvent-exchanging to clean DI-water was performed once for obtaining unstable CNC suspension before the solvent exchange step. Subsequently, the solvent exchange from water to acetone was carried out for removal of water and residual ions such as Na2+SO4− and Na+OH−.2.3. Chemical modification of CNCs
Modified protocol in solvent exchange method I proposed by Siqueira et al.31 was applied to the chemical modification process of CNCs, as described in Scheme 1b. Centrifugation (at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620g with 10 °C for 10 min) and redispersion (at 10
620g with 10 °C for 10 min) and redispersion (at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 s) were performed four times each exchange step to the desired solvent by using a high speed refrigerated centrifuge (Mega 17R, Hanil Science Industrial) and a homogenizer (Ultra Turrax® T25, IKA®), respectively. DMF and CF were our target solvents for the chemical modification and PLA film casting, respectively.
000 rpm for 30 s) were performed four times each exchange step to the desired solvent by using a high speed refrigerated centrifuge (Mega 17R, Hanil Science Industrial) and a homogenizer (Ultra Turrax® T25, IKA®), respectively. DMF and CF were our target solvents for the chemical modification and PLA film casting, respectively.
DMF suspension (100 ml) including CNCs of 1 g was added into a three-neck flat-bottomed flask with a reflux condenser under nitrogen condition, and the flask was immersed in silicon oil bath preheated up to 70 °C. After 30 min for obtaining stable DMF suspension at 70 °C, TDI was added into the suspension in drops with an equivalents ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for isocyanate groups in TDI and whole hydroxyl groups in CNCs. TDI grafting reaction was carried out for 24 h, and DMF exchanged to CF. Subsequently, CNC suspension in CF was sonicated by using sonicator (VCX 130, Sonics & Materials Inc.) to achieve homogeneous suspension for 15 min four times in an ice bath.
1 for isocyanate groups in TDI and whole hydroxyl groups in CNCs. TDI grafting reaction was carried out for 24 h, and DMF exchanged to CF. Subsequently, CNC suspension in CF was sonicated by using sonicator (VCX 130, Sonics & Materials Inc.) to achieve homogeneous suspension for 15 min four times in an ice bath.
2.4. Preparation of PLA nanocomposites
PLA nanocomposites reinforced CNCs or modified CNCs (mCNCs) were prepared by a doctor blade casting method. 3 g of PLA was dissolved in 20 ml CF suspension including dispersed CNCs or mCNCs (1–5 wt% compared to PLA weight) with a magnetic stirring (200 rpm) at room temperature for 24 h. The dissolved solution was casted onto a glass substrate with A4 size, and slow evaporation method32 was performed for 12 h. After the evaporation, the nanocomposites films were dried in convection oven at 70 °C for 24 h in order to eliminate residual solvent completely. Subsequently, the PLA film cooled down to room temperature was peeled from the glass substrate, and its thickness was approximately 50 μm. The PLA nanocomposite films with 0–5 wt% CNCs or mCNCs were coded as CNC0 to 5 or mCNC0 to 5, respectively.2.5. Characterizations
The size of pristine CNCs prepared by acid hydrolysis was examined using a transmission electron microscope (JEM 1400, JEOL) at a 120 kV accelerating voltage. 30 measurements of CNCs from a TEM image were randomly chosen using Image Pro Plus software (Media Cybernetics Inc.) to calculate average sizes in length and wide of obtained the CNCs. Fourier transformed infrared spectroscopy (FTIR, Nicolet™is™10 FT-IR Spectrometer, Thermo Scientific) with an attenuated total reflectance (ATR) accessory was used to characterize surface of the CNCs. The FTIR spectra were obtained from 16 scans with a resolution of 4 cm−1 under unforced condition. A background file was recorded prior to each scan at 4 cm−1 resolution with 16 scans, and all the samples (CNC and mCNC suspension in chloroform) were dried to completely remove the solvent at 70 °C for 3 h using a convection oven before the FTIR measurement. The tensile tests for the PLA nanocomposites films were conducted by using Universal Testing Machine (H50KS, Tinius Olsen) with a load cell of 500 N at a crosshead speed of 10 mm min−1 according to ASTM D 638. Sample dimension was 5 mm width and 70 mm length, but the thickness of each sample was measured prior to analysis. For assessment of optical property, UV-Vis spectrophotometer (UV-Vis, S-4100, Scinco) was employed to measure the transmittance of the samples over the spectral wave range from 200 nm to 700 nm. Thermal decomposition curves of all specimens were recorded by using thermal gravimetric analyzer (TGA, SDT Q600, TA instruments) with a heating rate of 10 °C min−1 under nitrogen atmosphere. The temperature range was scanned from 50 °C to 600 °C. Surface topography of CNC reinforced PLA film was explored using atomic force microscopy (AFM XE-10, Park systems) over an area of 225 μm2 with a scan rate of 0.3 Hz. Scanned side was on the surface of the PLA nano film in contact with the air, when the dissolved PLA solution was casted onto a glass substrate.3. Results and discussion
3.1. TDI modification of CNC surface
TEM image of CNCs isolated from conventional cellulose fibers is shown in Fig. 1. Needlelike nanocrystals with approximately 173 ± 45 nm length and 5.7 ± 0.9 nm wide are observed as reported elsewhere.16,18,33 TDI was introduced on the surface of the pristine CNCs to obtain stable suspension of CNCs in chloroform and to fortify bonding interaction with PLA matrix. Changes in FTIR spectra between CNCs and mCNCs are shown in Fig. 2, and notable peak changes can be observed in four specific band regions: (i) 1050–1125 cm−1 attributed to strong C–O stretching originated from primary and secondary alcohol (Fig. 2a); (ii) 2250–2275 cm−1 corresponding to N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (Fig. 2b); (iii) 1800–1200 cm−1 corresponding to C
O stretching (Fig. 2b); (iii) 1800–1200 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in amide group (Fig. 2c); (iv) 3000–3600 cm−1 corresponding to intermolecular hydrogen bonded O–H stretching (Fig. 2d).
O stretching in amide group (Fig. 2c); (iv) 3000–3600 cm−1 corresponding to intermolecular hydrogen bonded O–H stretching (Fig. 2d).
 | ||
| Fig. 2 FTIR-ATR spectra of CNCs and mCNCs: (a) full range of IR spectroscopy, (b) NCO region, (c) amide region, and (d) intermolecular hydrogen bonded OH region. | ||
As shown in Fig. 2a, the absorption band at 1100 cm−1 attributed to secondary alcohol is strongly increased after TDI modification of the CNCs compared with at near 1070 cm−1 corresponding to primary alcohol. From the result, it is suggested that reactivity of isocyanates prefers primary hydroxyl groups to secondary ones. This is because the relative reactivity of isocyanates against primary hydroxyl group is about three times as high as secondary hydroxyl group under condition of non-catalyst.34 In chemical modification of the CNCs using bifunctional TDI, our aim was to induce a chemical bond between the terminal carboxyl groups at end positions of PLA backbone and isocyanate groups on surface of mCNCs as well as physical interactions such as hydrogen bond and van der Waals forces, when incorporated with PLA resin. Fig. 2b shows distinctive change of band intensity between pristine CNCs and mCNCs at 2272 cm−1 corresponding to NCO stretching. The result can suggest the existence of the free second NCO group of the TDI after the modification of the CNCs because of reduction in reactivity of the second NCO group. It could be due to electron releasing effect of urethane group derived from the reaction between the hydroxyl group of the CNCs and the first NCO group of the TDI.34,35 Fig. 2c demonstrates clear evidence for mCNCs chemically linked with TDI. Several absorbance peaks are confirmed in the amide region: (i) at 1710 and 1537 cm−1 associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in urethane group, and (ii) 1600 cm−1 associated with C
O stretching in urethane group, and (ii) 1600 cm−1 associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in aromatic ring. In addition, intermolecular hydrogen bonding interaction between the mCNCs can be reduced due to TDI attachment onto surface of the mCNCs, as shown in Fig. 2d.
C stretching in aromatic ring. In addition, intermolecular hydrogen bonding interaction between the mCNCs can be reduced due to TDI attachment onto surface of the mCNCs, as shown in Fig. 2d.
Fig. 3 shows an effect of the surface modification on dispersion of the CNCs in CF. Significantly fast aggregation between CNC nanoparticles is occurred in CF despite just after sonication, on the other hand the suspension of mCNCs remains stable after time. The stability of mCNC nanoparticles in the hydrophobic organic solvent can be attributed to improving compatibility between the solvent and mCNC surface nature, which is induced by TDI.
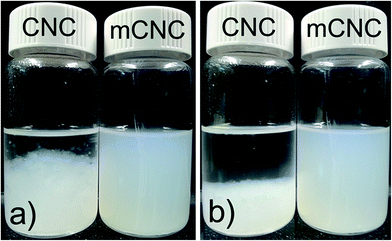 | ||
| Fig. 3 CNC and mCNC suspensions in chloroform: (a) after 15 min sonification and (b) after 15 min sonification and 5 min standing. | ||
3.2. Optical properties and roughness
Optical performance of polymer nanocomposites is one of key factors to assess dispersibility of nanoparticles in polymer matrix. Transparency test using the background image was performed to explore the dispersibility of CNCs and mCNCs in PLA matrix. Fig. 4 shows a clear difference in the transparency between the PLA films incorporated with CNCs and mCNCs with various nanocellulose contents (1, 3, and 5 wt%). The PLA films filled with the mCNCs show clear background images at even high loading level (5 wt%) of nanocellulose as well as a low loading level (1 wt%). On the other hand, the PLA nanofilms with the pristine CNCs are significantly opaque compared with mCNC cases. This is suggested that good distribution of the nanocellulose in the PLA matrix can be successfully obtained through TDI modification of the CNCs. | ||
| Fig. 4 Transparency of PLA films with CNCs (a–c) and mCNCs (d–f) test using background images as a function of nanocellulose loading level. | ||
UV-Vis analysis was performed for the more precise analysis on the optical property. Fig. 5 represents transmittance of PLA films with pristine CNCs (a) and mCNCs (b) in ultraviolet (UV) (100–400 nm) and visible light (400–700 nm) regions as a function of the nanocellulose content. The transparency of polymer films is generally gauged through measuring transmittance of visible light in the wavelength range of 540–560 nm according to ASTM-D1746-03 due to the eye sensitivity.36 As can be expected from Fig. 4, the PLA films with the mCNCs maintain higher transmittance than the CNC cases at the same content level in the range of 540–560 nm. Also, the PLA films with mCNCs reduce UV-B light in the range of 280–315 nm which is responsible for the photochemical degradation of packaged goods despite having higher transparency than the CNC case. Therefore, the transparent PLA film as a barrier against UV light can be obtained by incorporating TDI modified CNC due to homogeneity of the mCNCs in the PLA matrix.
 | ||
| Fig. 5 Transmittance of PLA films with CNCs (a) and mCNCs (b) in ultraviolet and visible light regions as a function of the nanocelluloses content. | ||
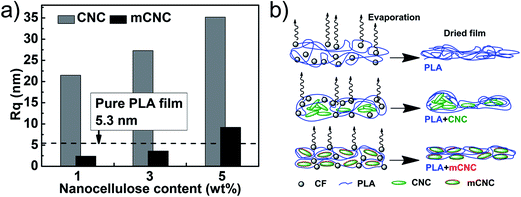
Qing et al.37 reported that the enhancement in the light transmittance of a film was attributed to increase in the surface smoothness (low roughness) of the film due to the reduction of surface light scattering. Thus, AFM measurement was performed to explore the surface roughness of PLA film with nanocellulose. Fig. 6 shows 3-D plotted AFM images representing the difference of the surface topography between the PLA films with CNCs (Fig. 6a: CNC1 and Fig. 6b: CNC5) and mCNCs (Fig. 6c: mCNC1 and Fig. 6d: mCNC5). The PLA films with mCNC have more even surface than CNC case, and the surface evenness reduces with increasing the loading level of the nanocellulose. To better understand the surface smoothness of the films, Rq (RMS roughness, root mean square roughness) values were calculated from the AFM images. Fig. 7a represents Rq values of PLA nanocomposites as a function of the nanocellulose content. Surface roughness increases with the nanocellulose loading level. Also, the Rq of the PLA films with the CNCs increases significantly in comparison with the mCNC cases. The Rq trend is good agreement with the trend of transmittance in the visible light. The increase of Rq can indicate the agglomeration occurrence of the nanoparticle in PLA matrix. The severe agglomeration of the CNCs in PLA matrix can be occurred due to the difference in the compatibility between the nanoparticle and PLA solution, and hence it is mandatory for the pristine CNCs case to have the Rq trend. On the contrary of the CNC case, the PLA films with mCNC have very low Rq values resulted from its good dispersibility in PLA matrix but show a similar Rq trend with CNC case as increasing nanocellulose loading level. The little agglomeration of the mCNC could be formed due to closed distance between mCNC particles with increasing mCNC loading level.
 | ||
| Fig. 6 Surface topography of PLA film with nanocelluloses in the area of 15 × 15 μm: (a) CNC1, (b) CNC5, (c) mCNC1, and (d) mCNC5. Height scale bar: 200 nm. | ||
Another noticeable thing is that the Rq values of PLA film with mCNC 1 and 3 are lower than that of the pure PLA film. Fig. 7b shows schematic illustration for demonstrating roughness changes of the pure PLA and PLA nanocomposite films. The polymer chains are experienced a stress relaxation caused by the chain entanglement when the solvent evaporation is occurred.38 The rate of solvent evaporation at each local evaporation point can be irregular and it may cause difference in the chain density. However, well-dispersed nanocrystals in PLA matrix reduce the difference in the density through decreasing the chain mobility.39 Therefore, the addition of mCNCs in the PLA matrix can make the surface of the PLA nanocomposite the smoother than the pure PLA film attributed to the improved dimensional stability of PLA network chain structure.
3.3. Thermal degradation behavior
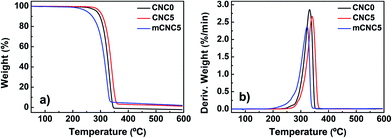
In general, thermal degradation behavior of composites incorporated with various materials is somewhat complex because the basic materials have each thermal degradation mechanism. Thus, examination of the basic components can be critical to understand the thermal properties of the final composite. Fig. 8 shows thermal decomposition behavior of CNCs and mCNCs. Degradation of the CNCs and mCNCs started at about 170 °C and 250 °C, respectively. Cellulose is mostly degraded between 250 °C and 400 °C through dehydration and depolymerization.40 The CNCs had a lower onset degradation temperature (<250 °C) than the cellulose. The CNCs were mostly produced through acid hydrolysis and the acid precipitates the dehydration of the CNCs.26,41 Since the CNCs had higher surface area as its size decreases to nanoscale, the heat transfer rate of the material is generally proportional to those surface areas. Thus it is inevitable to decrease the onset temperature of the thermal decomposition of acid hydrolyzed CNC. However, TDI chemical modification of the CNCs can overcome the drawback in the thermal stability because TDI onto CNC surface can disturb the dehydration taking place in a nanocellulose intramolecule.26 Therefore, mCNCs had higher thermal stability than the pristine CNCs. | ||
| Fig. 8 TGA results of CNCs and mCNCs: (a) weight loss, and (b) derivative weight as a function of temperature. | ||
Fig. 9 shows thermal gravimetric analysis of PLA nanocomposites with CNCs and mCNCs compared with neat PLA film. It is noticeable that the PLA film incorporated with the mCNCs shows lower thermal stability than the neat PLA film and PLA nanocomposite with pristine CNCs, although the thermal stability of the CNCs themselves was improved by introducing TDI onto its surface. The dispersibility improvement of the mCNCs in the PLA matrix results in its high surface area compared with the agglomerated CNCs, and hence the mCNCs can reduce thermal stability of the PLA film with the mCNCs due to the increase of the heat transfer rate in the PLA film, as explained above. Therefore, the addition of a flame retardant may be necessary to achieve the high thermal stability of the PLA nanocomposite reinforced with the mCNCs.
 | ||
| Fig. 9 TGA results of PLA films with CNC and mCNCs: (a) weight loss, and (b) derivative weight as a function of temperature. | ||
3.4. Mechanical properties
Effects of CNCs and mCNCs reinforcement on the tensile properties of PLA films are represented in Fig. 10 as a function of CNC loading level. The tensile strength of the PLA film with the pristine CNCs linearly decreased with increasing the CNC loading level. However, the mCNC case shows a fluctuation trend in the strength with increasing the mCNC loading level, and the PLA film with mCNCs has the maximum tensile strength (about 59.3 MPa) at the 1 wt% mCNC loading level. The reduction in the strength with the nanocellulose loading level can be mainly due to the agglomeration phenomenon between the nanoparticles, as explained in the roughness section. Our previous study42 showed that the agglomeration of the particle decreased mechanical properties of its composites due to creating the void between the particle and the matrix. The increase of the tensile strength in the mCNC filled PLA film until reached at the maximum strength can be attributed to the better dispersibility of the mCNCs in the PLA matrix. | ||
| Fig. 10 Effects of CNCs and mCNCs on tensile properties ((a): strength, and (b): elongation modulus) of PLA nanocomposites. | ||
On the basis of the FTIR results, TDI moieties attached onto mCNC surface by urethane linkage possibly have various interactions with the PLA backbone. Scheme 2 shows the schematic illustrations of the interactions existing at the interfaces between mCNC and the PLA backbone. A toluene moiety of the mCNC and a methyl group of the PLA backbone can form van der Waals force, and various hydrogen bonds (N⋯H and O⋯H) can be occurred. Also, it can be possible to generate a chemical crosslinking through forming amide group by the reaction between isocyanates of TDI and terminal hydroxyl or carboxyl groups of the PLA backbone. Therefore, these interactions as well as the distribution of the mCNC can be helpful for improving mechanical properties of the PLA matrix.
 | ||
| Scheme 2 Schematic illustration of the dispersibility of mCNC in PLA matrix and interfacial interactions between mCNC and PLA backbone. | ||
In the case of tensile modulus (Fig. 10b), both CNC and mCNC cases show no considerable changes with increasing the nanocellulose content level. Also, the PLA films filled with the mCNCs have higher tensile modulus (elongation modulus) than the pristine CNC cases. The increase of the tensile modulus can be associated with the increased stiffness of the composite materials.43 The stiff CNCs can act as hard domain as forming homogenous phase in the soft amorphous PLA matrix through the TDI chemical modification. This is because TDI modification of the CNCs leads enhancement of the compatibility between the CNCs and PLA matrix, as explained above. Hence, the good dispersion of the mCNC can improve stiffness of the soft PLA matrix. However, the function as stiff hard domain might be offset by little agglomeration of the mCNCs as increasing mCNC loading level. Therefore, the PLA film with the mCNCs has higher modulus than the pure PLA film and the PLA film with the CNCs, but the increase of the mCNC content has nearly influence on improving the modulus of the PLA film with the mCNCs.
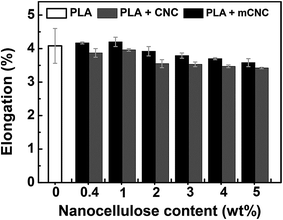
Fig. 11 shows the elongation of the PLA nanocomposites as a function of the nanocellulose loading level. It has similar trends with the tensile strength and modulus in comparison between PLA films with CNCs and mCNCs. This trend can be due to formation of a continuous structure of mCNC in PLA matrix by enhancing compatibility between them, as explained above.
4. Conclusions
Chemical modification of cellulose nanocrystals prepared by sulfuric acid hydrolysis was performed using toluene diisocyanate, and the nanocellulose was successfully chemically-modified with TDI. The modified CNCs (mCNCs) were analyzed by FTIR spectroscopy and it provided clear evidences of transformation in the surface structure, and stable mCNC suspension in chloroform was achieved. In addition, analyses of AFM images and UV-Vis spectroscopy showed a good distribution of the mCNCs in PLA matrix. From TGA results, the TDI modification of the CNCs remarkably improved the thermal stability of the mCNCs, but the thermal stability of the PLA films with mCNCs somewhat reduced due to the dispersibility enhancement of mCNCs. Tensile properties of the PLA films with the mCNCs mostly increased in comparison with both of the neat PLA film and CNC filled PLA films attributed to existence of various interaction forces between the PLA matrix and mCNCs and to a good distribution in the PLA matix. Therefore, TDI modification of the CNCs is a good approach to overcome the drawback representing in organic solvent system and to improve physical and mechanical properties of the neat PLA film.References
- H. P. S. A. Khalil, Y. Davoudpour, M. N. Islam, A. Mustapha, K. Sudesh, R. Dungani and M. Jawaid, Carbohydr. Polym., 2014, 99, 649–665 CrossRef PubMed.
- Y. Habibi, Chem. Soc. Rev., 2014, 43, 1519–1542 RSC.
- K. H. Choi, S. J. Cho, S. J. Chun, J. T. Yoo, C. K. Lee, W. Kim, Q. Wu, S. B. Park, D. H. Choi, S. Y. Lee and S. Y. Lee, Nano Lett., 2014, 14, 5677–5686 CrossRef CAS PubMed.
- S. J. Chun, E. S. Choi, E. H. Lee, J. H. Kim, S. Y. Lee and S. Y. Lee, J. Mater. Chem., 2012, 22, 16618–16626 RSC.
- S. F. Anis, B. S. Lalia and R. Hashaikeh, Desalination, 2014, 336, 138–145 CrossRef CAS.
- J. T. Korhhonen, M. Kettunen, R. H. A. Ras and O. Ikkala, ACS Appl. Mater. Interfaces, 2011, 3, 1813–1816 Search PubMed.
- S. Y. Lee, S. J. Chun, I. A. Kang and J. Y. Park, J. Ind. Eng. Chem., 2009, 15, 50–55 CrossRef CAS.
- Z. Zhang, Q. Wu, K. Song, S. Ren, T. Lei and Q. Zhang, ACS Sustainable Chem. Eng., 2015, 3, 574–582 CrossRef CAS.
- S. Spinella, G. L. Re, B. Liu, J. Dorgan, Y. Habibi, P. Leclere, J. M. Raquez, P. Dubois and R. A. Gross, Polymer, 2015, 65, 9–17 CrossRef CAS.
- K. Benhamou, H. Kaddami, A. Magnin, A. Dufresne and A. Ahmad, Carbohydr. Polym., 2015, 122, 202–211 CrossRef CAS PubMed.
- L. Petersson, I. Kvien and K. Oksman, Compos. Sci. Technol., 2007, 67, 2535–2544 CrossRef CAS.
- A. Pei, Q. Zhou and L. A. Berglund, Compos. Sci. Technol., 2010, 70, 815–821 CrossRef CAS.
- S. Y. Lee, D. J. Mohan, I. A. Kang, G. H. Doh, S. Lee and S. O. Han, Fibers Polym., 2009, 10, 77–82 CrossRef CAS.
- G. Siqueira, J. Bras, N. Follain, S. Belbekhouche, S. Marais and A. Dufresne, Carbohydr. Polym., 2013, 91, 711–717 CrossRef CAS PubMed.
- K. Missoum, J. Bras and M. N. Belgacem, Cellulose, 2012, 19, 1957–1973 CrossRef CAS.
- L. Rueda, B. F. d'Arlas, Q. Zhou, L. A. Berglund, M. A. Corcuera, I. Mondragon and A. Eceiza, Compos. Sci. Technol., 2011, 71, 1953–1960 CrossRef CAS.
- G. Siqueira, J. Bras and A. Dufresne, Langmuir, 2010, 26, 402–411 CrossRef CAS PubMed.
- M. D. O. Taipina, M. M. F. Ferrarezi, I. V. P. Yoshida and M. D. C. Goncalves, Cellulose, 2013, 20, 217–226 CrossRef.
- J. M. Raquez, Y. Murena, A. L. Goffin, Y. Habibi, B. Ruelle, F. DeBuyl and P. Dubois, Compos. Sci. Technol., 2012, 72, 544–549 CrossRef CAS.
- A. N. Frone, S. Berlioz, J.-F. Chailan, D. M. Panaitescu and D. Donescu, Polym. Compos., 2011, 32, 976–985 CrossRef CAS.
- J. Lu, P. Askeland and L. T. Drzal, Poymer, 2008, 49, 1285–1296 CAS.
- S. J. Chun, S. Y. Lee, G. Y. Jeong and J. H. Kim, J. Ind. Eng. Chem., 2012, 18, 1122–1127 CrossRef CAS.
- N. Follain, M.-F. Marais, S. Montanari and M. R. Vignon, Polymer, 2010, 51, 5332–5344 CrossRef CAS.
- N. Lin, J. Huang, P. R. Chang, J. Feng and J. Yu, Carbohydr. Polym., 2011, 83, 1834–1842 CrossRef CAS.
- N. Lin, G. Chen, J. Huang, A. Dufresne and P. R. Chang, J. Appl. Polym. Sci., 2009, 113, 3417–3425 CrossRef CAS.
- H. Rosilo, E. Kontturi, J. Seitsonen, E. Kolehmainen and O. Ikkala, Biomacromolecules, 2013, 14, 1547–1554 CrossRef CAS PubMed.
- E. Kloser and D. G. Gray, Langmuir, 2010, 26, 13450–13456 CrossRef CAS PubMed.
- Z. Duan and N. L. Thomas, J. Appl. Phys., 2014, 115, 064903 CrossRef.
- W. K. Chee, N. A. Ibrahim, N. Zainuddin, M. F. A. Rahman and B. W. Chieng, Adv. Mater. Sci. Eng., 2013, 2013, 1–8 CrossRef.
- J. Majoinen, A. Walther, J. R. McKee, E. Kontturi, V. Aseyev, J. M. Malho, J. Ruokolainen and O. Ikkala, Biomacromolecules, 2011, 12, 2997–3006 CrossRef CAS PubMed.
- G. Siqueira, J. Bras and A. Dufresne, Langmuir, 2010, 26, 402–411 CrossRef CAS PubMed.
- Z. G. Tang, R. A. Black, J. M. Curran, J. A. Hunt, N. P. Rhodes and D. F. Williams, Biomaterials, 2004, 25, 4741–4748 CrossRef CAS PubMed.
- M. C. Li, Q. Wu, K. Song, S. Lee, Y. Qing and Y. Wu, ACS Sustainable Chem. Eng., 2015, 3, 821–832 CrossRef CAS.
- M. Lonescu, Chemistry and technology of polyols for polyurethanes, RapraTechnology Ltd., Shrewsbury, 1st edn, 2005 Search PubMed.
- J. G. Gwon, S. K. Kim and J. H. Kim, J. Porous Mater., 2015 DOI:10.1007/s 10934-015-0100-0.
- S. Ligot, S. Benali, R. Ramy Ratiarson, M. Murariu, R. Snders and P. Dubois, Mater. Sci. Adv. Res., 2015, 1, 20–30 Search PubMed.
- Y. Qing, Z. Cai, Y. Wu, C. Yao, Q. Wu and X. Li, Ind. Crop. Prod., 2015, 77, 13–20 CrossRef CAS.
- K. S. Tiaw, S. H. Teoh, R. Chen and M. H. Hong, Biomacromolecules, 2007, 8, 807–816 CrossRef CAS PubMed.
- A. J. de Menezes, G. Siqueira, A. A. S. Curvelo and A. Dufresne, Polymer, 2009, 50, 4552–4563 CrossRef CAS.
- S. Liodakis, D. Bakirtzis and A. P. Dimitrakopouos, Thermochim. Acta, 2003, 399, 31–42 CrossRef CAS.
- M. Roman and W. T. Winter, Biomacromolecules, 2004, 5, 1671–1677 CrossRef CAS PubMed.
- J. G. Gwon, S. Y. Lee, S. J. Chun, G. H. Doh and J. H. Kim, J. Compos. Mater., 2011, 46, 301–309 CrossRef.
- S. Y. Lee, S. J. Chun, G. H. Doh, I. A. Kang, S. Lee and K. H. Park, J. Compos. Mater., 2009, 43, 1639–1657 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |