Versatile boiler ash containing potassium silicate for the synthesis of organic carbonates†
Vidhyaa Paroo Indrana,
Anisah Sajidah Haji Sauda,
Gaanty Pragas Maniamab,
Mashitah Mohd. Yusoffa,
Yun Hin Taufiq-Yapc and
Mohd Hasbi Ab. Rahim*ad
aFaculty of Industrial Sciences and Technology, Universiti Malaysia Pahang, Lebuhraya Tun Razak, 26300 Kuantan, Pahang, Malaysia. E-mail: mohdhasbi@ump.edu.my
bCentral Laboratory, Universiti Malaysia Pahang, Lebuhraya Tun Razak, 26300 Kuantan, Pahang, Malaysia
cCatalysis Science and Technology Research Centre, Faculty of Science, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia
dCentre for Earth Resources Research & Management, Universiti Malaysia Pahang, Lebuhraya Tun Razak, 26300 Kuantan, Pahang, Malaysia
First published on 24th March 2016
Abstract
In this study, boiler ash containing potassium silicate (BA 900) and potassium silicate (K2SiO3) were proven to be feasible Lewis acid catalysts for the synthesis of different organic carbonates (glycerol carbonate, ethylene carbonate, and propylene carbonate) from different polyol (glycerol, ethylene glycol, and propylene glycol) feedstocks. In addition, the developed catalytic reaction has the ability to produce propylene carbonate at milder reaction temperatures. BA 900 and K2SiO3 were reusable for three consecutive reaction cycles without the loss of activity. The reusable characteristics of catalysts were confirmed through several characterisation techniques, i.e. XRD, FTIR, XRF, N2 physisorption, FESEM-EDX, and Hammett test. All organic carbonates synthesised had a similar synthetic mechanistic pathway, which involved decomposition of intermediate carbamates into their respective carbonates.
Introduction
In our previous report, it was found that the boiler ash from the palm oil industry was introduced as a catalyst for the synthesis of glycerol carbonate (GC) from glycerol and urea as a bio-renewable feedstock.1 In the present study, the feasibility of the catalyst to synthesise ethylene carbonate (EC) and propylene carbonate (PC) is discussed. These specific carbonates were chosen due to their adverse use in many industries. Ethylene carbonate and propylene carbonate have variety of applications in many major industries such as polymers, gas separation membranes, paints, agrochemicals, batteries as well as supercapacitors.2–4 Apart from that, the previously identified element in boiler ash, i.e. potassium silicate (K2SiO3), that was responsible for the catalytic behaviour of boiler ash was also introduced as a new catalyst for the synthesis of GC. There are various routes of synthesising GC. One of the very commonly known routes is through direct carbonylation of glycerol using carbon dioxide.5 This reaction is conducted at higher temperatures of 180 °C with pressures of 5 MPa. However, this catalytic process is less feasible due to low conversions of glycerol as well as poor yields of GC due to the stability of CO2. Besides, the direct carbonylation reaction requires very high energy input and a very effective catalyst for conversion to occur.6 Another way of synthesising GC is through the use of glycerol and dimethyl carbonate (DMC) with the aid of catalyst. Even though this synthesis route is often employed by many, the reaction requires higher ratios of DMC to glycerol and it also causes shifts in the chemical equilibrium.7 A recent synthesis proposes the use of glycerol and urea as the feedstocks for the synthesis of GC.8,9 The use of relatively cheaper bio-renewable feedstocks and catalysts derived from industrial waste has gained our interest in conducting the study. On the other hand, previous studies conducted by our group using gypsum in the glycerolysis reaction with urea to produce glycerol carbonate also suggests proper utilisation of industrial waste.10 Though there have been studies reported on the use of catalysts containing K-zeolite derived from fly ash for the transesterification reaction of glycerol with DMC, catalyst preparation involved deposition of synthetically derived potassium ions requiring several pre-treatment stages.11 Therefore, waste boiler ash is deduced herein as a catalyst for a greener and more cost effective reaction operation. Similar to glycerol being used as a feedstock,1 the cyclisation of ethylene glycol and propylene glycol to form their respective carbonates was possible through the use of boiler ash as a catalyst and it was reported for the first time herein.Results and discussion
Catalytic mechanism of potassium silicate contained in boiler ash for the synthesis of glycerol carbonate
Based on our previous study, potassium silicate (K2SiO3) contained in boiler ash was found to be the major element that influenced the catalytic behaviour of boiler ash in synthesising GC from glycerol and urea. The presence of potassium silicate in boiler ash was verified using XRD analysis1 as well as X-Ray Fluorescence (XRF) data reported herein. Therefore, the detailed catalytic mechanism of potassium silicate contained in boiler ash for the synthesis of GC is proposed as follows. It can be inferred that the K+ ion acts as a weak Lewis acid by activating the carbonyl group of urea. On the other hand, SiO32− acts an effective conjugate basic site to activate the hydroxyl group of glycerol. Based on Scheme 1, the activation of the carbonyl group of urea grounds the carbonyl group to become positively charged, further activating it as an electrophile. In contrast, the activated hydroxyl group of glycerol acts as a nucleophile. In step 1, nucleophilic attack by the hydroxyl group towards the electrophilic carbonyl of urea forms the carbamate intermediate while releasing ammonia gas. It is believed that the second step is the most anticipated reaction phase where the selectivity towards the desired product formation is evidently observed due to the influence of the catalyst. The cyclisation is complete by the loss of more ammonia gas, followed by ring formation and rearrangement of the carbonyl double bond to form GC.Reusability study of BA 900 and K2SiO3
In any fine chemical synthesis industry, the reusability of catalyst is one of the crucial parameters of concern. Thus, both boiler ash (BA 900) and K2SiO3 were subjected to reusability tests carried out under standard reaction conditions. As depicted in Fig. 1 and 2, the conversion, selectivity and yield were significantly comparable and showed optimum performance in all three cycles for both tested catalysts. The reusability of the K2SiO3 catalyst is anticipated since the fresh and spent catalyst exhibits comparable characteristics through analysis conducted using FTIR, XRD, FESEM and N2 physisorption.However, unobserved FTIR peaks in the 1200–1500 cm−1 range for spent catalyst was found due to the presence of glycerol moieties that remained intact on the catalyst that then interfered and broadened the FTIR signal (Fig. 3). For the record, the spent catalyst was subjected to washing only with deionised water, thus remaining glycerol is expected. The slight difference in the XRD pattern, as shown in Fig. 4, is due to the dominant presence of SiO2 in the spent catalyst as an evident from EDX analysis (Table 1). More dominant amorphous SiO2 is expected to be present due to the loss of some potassium ions, thus the XRD diffractogram will closely resemble the diffraction pattern of pure SiO2.12
| Element | wt% (fresh) | wt% (spent) |
|---|---|---|
| O | 56.11 | 57.27 |
| Si | 26.01 | 28.47 |
| K | 17.88 | 14.26 |
| Totals | 100.00 | 100.00 |
Based on the analysis being carried out using N2 physisorption, it was found that the BET surface area of both fresh and spent K2SiO3 were comparable (Table 2). However, spent catalyst contains larger pore volumes and pore sizes. This scenario is expected to occur due to the loss of some potassium ions embedded to the silicate, thus resulting in larger silicate pores to be present. As mentioned, it was found that potassium ions and silicate ions play an important role in the catalytic mechanism. Therefore, the variation of catalyst surface area, porous structure and morphology (Fig. 5) are less significant. The presence of K2SiO3 predominantly in calcined boiler ash (BA 900) was verified using XRF analysis (Table 3). It was also found that at higher heat treatments under a static air environment, KCl that existed in dried boiler ash is later transformed into K2SiO3 where the loss of the chlorine ion can be expected, as reported by our group1 (ESI 1†). The presence of other elements such as MgO, CaO, Al2O3, Fe2O3, P2O5, ZrO2 are at minor levels that do not significantly affect the catalytic activity of boiler ash. In addition, the presence of SiO2 also does not affect the catalytic activity of boiler ash and gives comparable GC yields to the uncatalysed reaction.1 Indeed, it was found that potassium ions along with its conjugated basic site (i.e. KCl, KOH and K2SiO3) acted as the most effective metal element of interest. Apart from that, the XRF analysis conducted was in agreement with the ICP-MS and FESEM-EDX analysis reported earlier, in which the dominant metal element detected in all three analysis was potassium.1
| Catalyst | BET surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore size (Å) |
|---|---|---|---|
| Fresh | 2.50 | 0.003 | 45.14 |
| Spent | 2.54 | 0.021 | 342.06 |
| Analyte | Mass% |
|---|---|
| K2SiO3 | 43.53 |
| SiO2 | 33.14 |
| K2O | 7.17 |
| CaO | 8.98 |
| MgO | 2.41 |
| Al2O3 | 1.93 |
| Fe2O3 | 1.89 |
| P2O5 | 0.73 |
| ZrO2 | 0.22 |
| Total | 100 |
Even though K2SiO3 demonstrated reusable properties, only 56.3 wt% of the catalyst was recovered after the third cycle. The partial recovery of the catalyst might be due to the delocalisation of potassium ions during the reaction and washing procedure as well as contribution from systematic and random error during the experimental work. Indeed, the minor amount of metal leached was confirmed through Energy Dispersive X-ray (EDX) analysis of spent catalyst and ICP-MS analysis of the reaction solution, where 2.4% K and 18.21% of Si were lost.
In similar trend to K2SiO3, only 31.7 wt% of catalyst was recovered after the third cycle. The higher weight percent of catalyst loss observed from BA 900 compared to K2SiO3 can be attributed to the loss of other present elements in boiler ash, as previously reported1 apart from leaching of potassium ions. It is also notable that the FTIR spectrum (Fig. 6) of spent BA 900 and spent K2SiO3 showed an almost similar pattern. This qualitative datum indicates that the major composition of boiler ash predominantly exists in the potassium silicate phase. Besides, there is also no significant difference between the FTIR spectra (Fig. 7) of fresh BA 900 and spent BA 900. Moreover, the Hammett test from Table 4 also indicates the basic property of all fresh and spent BA 900, as well as K2SiO3, as confirmed by colour changes in the phenolphthalein indicator. Hence, the activity of reusable boiler ash is expected to be comparable to reusable K2SiO3. Indeed, the calculated turn over frequency (TOF) of K2SiO3 is 125.6 mmol per g cat per h, and did not deviate from the TOF value of BA 900(126.5 mmol per g cat per h).
| Catalyst | Methyl red (H = 4.8) | Phenolphthalein (H = 8.2) | 2,4-Dinitroaniline (H = 15) | 4-Nitroaniline (H = 18.4) |
|---|---|---|---|---|
| Fresh BA 900 | No change | Colourless to pink solution | No change | No change |
| Spent BA 900 | No change | Colourless to pink solution | No change | No change |
| Fresh K2SiO3 | No change | Colourless to pink solution | No change | No change |
| Spent K2SiO3 | No change | Colourless to pink solution | No change | No change |
Catalyst feasibility study for the synthesis of ethylene carbonate and propylene carbonate
Waste boiler ash (BA 900) was used as the catalyst for a greener and more cost effective reaction operation. Similar to glycerol as a feedstock,1 the cyclisation of ethylene glycol and propylene glycol to form their respective carbonates was made possible through the use of boiler ash catalyst and it is reported for the first time herein. Potassium silicate (K2SiO3) contained in boiler ash could aid in the selective carbonylation of ethylene glycol and propylene glycol to their respective carbonates. As shown in Table 5, the optimum yield of EC of 80.1 ± 0.6% was obtained after 8 h reaction time while at 10 h, the yield of PC was 73.8 ± 0.7%. It is important to note that catalytic activity for both EC and PC show almost identical TOF, indicating that the amount of active species responsible for the transcarbonylation reaction is equal. Apart from that, the reaction involving the use of K2SiO3 alone instead of boiler ash produced almost similar yields of the respective carbonates of EC (79.9 ± 0.3%) and PC (72.8 ± 0.7%). Thus, it is suggested that the catalytic activity was due to the presence of K+, which acted as a weak Lewis acid to activate urea while the conjugate basic site SiO32− activated the hydroxyl group of the polyols to form the respective carbonates. Moreover, the current study proposes better yields of EC than a previously reported study.13,14 Besides, the previous study reported the synthesis of PC being operated at a higher temperature (170 °C and 180 °C), which could contribute to a relatively non-cost effective process.15–17 Indeed, the use of higher temperatures over 150 °C was not favoured due to the reported decomposition of urea into isocyanic acid.18 It is worth noting that there is no available study being reported on the use of potassium as an active catalyst for the cyclisation of polyols with the aid of urea.| No. | Reaction/catalyst | Temperature (°C) | Molar ratio of polyol to urea | Conversion of polyol | Duration (h) | Yield% of organic carbonate | TOF (mmol per g cat per h) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: catalyst mass, 0.25 g; gas, N2; standard stirring rate, 340 rpm; TOF: calculated based on the yield of carbonate multiplied by mmol of initial polyols per gram catalyst per total reaction time (h). Quantitative analysis was carried out using an Agilent Technologies A7890 Gas Chromatography-Flame Ionised Detector (GC-FID) equipped with a DB-WAX (60 m, 0.25 mm, 0.25 μm) column. | |||||||
| 1 | Ethylene carbonate | 150 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol 1 ethylene glycol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea urea |
60.5 | 8 | 17.9 | — |
| Blank | |||||||
| BA 900 | 92.7 | 8 | 80.1 | 120.2 | |||
| K2SiO3 | 92.7 | 8 | 79.9 | 119.8 | |||
| 2 | Propylene carbonate | 150 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 propylene glycol 1 propylene glycol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea urea |
78.4 | 10 | 36.3 | — |
| Blank | |||||||
| BA 900 | 89.2 | 10 | 73.2 | 117.1 | |||
| K2SiO3 | 89.4 | 10 | 72.8 | 116.5 | |||
Proposed mechanistic pathway of EC and PC using boiler ash as a catalyst
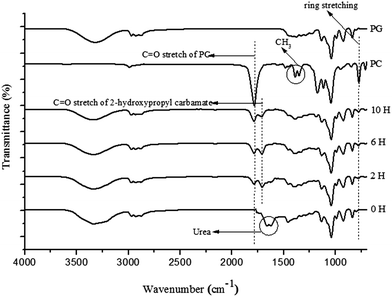
The cyclisation pathway of ethylene glycol and propylene glycol, along with their by-product formation, using boiler ash as a catalyst is proposed in Schemes 2 and 3. The mechanistic pathway for both EC and PC through the respective carbamate intermediate was confirmed via time online studies (TOL) monitored by ATR-FTIR (Fig. 8 and 9) and 13C NMR (ESI 2†). Both FTIR spectra showed the presence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1715 cm−1) group of 2-hydroxyethyl carbamate and 2-hydroxypropyl carbamate, which later on transformed into carbonate compounds. There was also no sign of the N–C–O stretching of isocyanic acid in peak observation at 2210 cm−1, which indicates that the reaction is solely dependent on carbamate as an intermediate compound. In Fig. 8, the band at 1801 cm−1 and 1788 cm−1 indicate the symmetrical C
O (1715 cm−1) group of 2-hydroxyethyl carbamate and 2-hydroxypropyl carbamate, which later on transformed into carbonate compounds. There was also no sign of the N–C–O stretching of isocyanic acid in peak observation at 2210 cm−1, which indicates that the reaction is solely dependent on carbamate as an intermediate compound. In Fig. 8, the band at 1801 cm−1 and 1788 cm−1 indicate the symmetrical C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of EC.19 The ring stretching peak of EC and PC were visible as the duration of the reaction is increased and was present in the 770–785 cm−1 range. In case of TOL of PC, the methyl group was also observed to be formed at 1352 cm−1 and 1380 cm−1. The decomposition of urea from 0 h to 10 h is visible from being consumed in the reaction where the peak for urea, labelled in Fig. 8 and 9, gradually disappeared.
O stretching of EC.19 The ring stretching peak of EC and PC were visible as the duration of the reaction is increased and was present in the 770–785 cm−1 range. In case of TOL of PC, the methyl group was also observed to be formed at 1352 cm−1 and 1380 cm−1. The decomposition of urea from 0 h to 10 h is visible from being consumed in the reaction where the peak for urea, labelled in Fig. 8 and 9, gradually disappeared.
Based on TOL analysis conducted using ATR-FTIR and 13C NMR, the mechanistic pathway for both EC and PC corresponds to the similar pathway observed for GC, where the formation of GC originates through the selective transformation of the respective carbamate intermediate. Even though a similar route was observed in related literature,15,20 a slight difference was perceived with boiler ash as the catalyst whereby the conversion of carbamate into the respective carbonate occurred in an accelerated manner. However, some of the by-products detected in prior experiments were not detected in this study, such as biuret, which is also known as carbamylurea.13 Similar findings on the reaction pathway involved the decomposition of intermediate carbamate into EC.13,20 On the other hand, Wang and co-researchers in 2014 proposed the formation of PC was due to the decomposition of an intermediate 2-hydroxypropyl carbamate, which was similar to the current findings.15 However, they also reported the presence of isocyanic acid, which later forms the intermediate compound. This scenario is expected since the reaction was carried out at temperatures over 150 °C, thus increasing the possibility of urea to decompose into isocyanic acid. Schemes 2 and 3 illustrate the reaction pathway of EC and PC using BA 900 containing potassium silicate as a catalyst.
Experimental
Materials
Glycerol (99.5%) and Urea (AR Grade) were purchased from Friendemann Schmidt Chemical. Waste boiler ash, used as the catalyst, was collected from a palm oil mill located in Lepar Hilir, Pahang, Malaysia. The boiler ash used is the ash obtained from incineration of palm fruits, palm kernel, palm shells and palm fibre. Potassium silicate (K2SiO3) ≥99.9% was purchased from Sigma-Aldrich.Catalyst preparation
Raw boiler ash was dried at 110 °C for overnight and then powdered using a mortar and pestle. Then, the ash was sieved using a 200 μm sized sieve. Moreover, 2 g of the sieved ash was loaded on the combustion boat and calcined under static air at a temperature of 900 °C for 4 h in a horizontal tube furnace with a temperature ramping rate of 5 °C min−1. The catalysts were later denoted as BA 900. Potassium silicate was directly used without any pre-treatment.Catalytic testing
The reaction of EC and PC synthesis was carried out using a three-neck round bottom flask attached to a cycle condenser with a water cooling system. The reaction parameters are as described in Table 5. For the reaction involving synthesis of EC, 18.6 g of ethylene glycol was allowed to heat up to 150 °C for 10 h under a flow of nitrogen gas for 20 min before adding urea and catalyst, while the temperature and stirring rate was controlled using an IKA@DTS-5 temperature controller. When the temperature reached 150 °C, 12.0 g urea and 0.25 g catalyst were added to the reaction and it was stirred using a magnetic stirrer at a rate of 340 rpm. The molar ratio of ethylene glycol to urea used was (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For the reaction involving synthesis of PC, 30.4 g of propylene glycol was allowed to heat up to 150 °C for 10 h under the flow of nitrogen gas for 20 min before adding urea and catalyst while the temperature and stirring rate was controlled using an IKA@DTS-5 temperature controller. When the temperature reached 150 °C, 6.0 g urea and 0.25 g catalyst were added to the reaction and stirred using magnetic stirrer at a rate of 340 rpm. The molar ratio of propylene glycol to urea used was (4
1). For the reaction involving synthesis of PC, 30.4 g of propylene glycol was allowed to heat up to 150 °C for 10 h under the flow of nitrogen gas for 20 min before adding urea and catalyst while the temperature and stirring rate was controlled using an IKA@DTS-5 temperature controller. When the temperature reached 150 °C, 6.0 g urea and 0.25 g catalyst were added to the reaction and stirred using magnetic stirrer at a rate of 340 rpm. The molar ratio of propylene glycol to urea used was (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Sampling was done from 0 h to 10 h with time intervals of 1 h. 50 μl of sample was transferred into 1450 μl of deionised water. The experiment was repeated three times for the repeatability study.
1). Sampling was done from 0 h to 10 h with time intervals of 1 h. 50 μl of sample was transferred into 1450 μl of deionised water. The experiment was repeated three times for the repeatability study.
The reaction setup was similar to the reaction of EC and PC for the reusability study of potassium silicate (K2SiO3). Glycerol was heated up to 150 °C for 20 min under the flow of nitrogen gas before adding urea and catalyst. The molar ratio of glycerol to urea was (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5) and 0.5 g catalyst (K2SiO3) was added to the reaction and the sampling was done at 4 h. 50 μl of sample was transferred into 1450 μl of deionized water. The reaction mixture was immediately placed into a 100 ml centrifuge tube and centrifuged for 1 h at 8000 rpm. The catalyst deposited as residue on the bottom of the centrifuge tube was carefully transferred into a clean 100 ml centrifuge tube and washed with deionized water. The water was removed from the centrifuge tube after 30 min of centrifugation. This step was repeated 4 times. The final catalyst was filtered and dried overnight at 110 °C and the synthesis of GC using dried catalyst was repeated for the second cycle. The same procedure was again repeated for the third cycle.
1.5) and 0.5 g catalyst (K2SiO3) was added to the reaction and the sampling was done at 4 h. 50 μl of sample was transferred into 1450 μl of deionized water. The reaction mixture was immediately placed into a 100 ml centrifuge tube and centrifuged for 1 h at 8000 rpm. The catalyst deposited as residue on the bottom of the centrifuge tube was carefully transferred into a clean 100 ml centrifuge tube and washed with deionized water. The water was removed from the centrifuge tube after 30 min of centrifugation. This step was repeated 4 times. The final catalyst was filtered and dried overnight at 110 °C and the synthesis of GC using dried catalyst was repeated for the second cycle. The same procedure was again repeated for the third cycle.
Product analysis
A GC-FID Agilent Technologies 7890A equipped with a DB-WAX (60 m, 0.25 mm, 0.25 μm) column was used to analyse the product of EC and PC. Helium gas was used as the carrier gas with a flow rate of 1.5 ml min−1. The temperature of the injector and the detector were 225 °C and 250 °C, respectively. The temperature of the column was programmed to have a 2 min initial hold at 80 °C and then 15 °C min−1 ramp from 80 °C to 250 °C at 5 min holding time. The split ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and the injection volume was 1 μl.
10 and the injection volume was 1 μl.
An ATR-FTIR Perkin Elmer USA was used to study the functional groups present in the online time analysis of the product from 0 min to 10 h, which could attribute to the product and by products present in the reaction mixture as a validation. A single drop of reaction mixture was placed on the liquid holder and the transmission data was collected in the 4000–700 cm−1 range. NMR on a BRUKER Ultra Shield Plus 500 MHz was also used to study the 13C NMR of the products formed.
An Inductive Coupled Plasma-Mass Spectrometer (ICP-MS) Agilent 7500c was used to study the potassium and silicate content in the reaction mixture using an in-house method of CHEMITEL/WI/CHEMTM/001 that was based on AOAC999.10.
Catalyst characterisation
A XRD Rigaku Miniflex II was used to analyse the XRD pattern of the fresh and spent K2SiO3. Diffraction patterns were recorded with Cu Kα radiation (λ = 1.5406 A), which was operated at 40 kV and 30 mA over the range of 3 to 80° at 2 theta (θ) for crystalline phase determination at a scan speed of 0.04 degree per s. The catalyst samples in powder form were then loaded individually on a glass sample holder for measurement.FTIR transmission data were collected from a pressed catalyst disk made with KBr with a ratio of (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) catalyst to KBr in the 4000–400 cm−1 scanning range for fresh and spent BA 900 and K2SiO3.
10) catalyst to KBr in the 4000–400 cm−1 scanning range for fresh and spent BA 900 and K2SiO3.
A Shimadzu X-ray fluorescence 720 was used to analyse metal oxide content in boiler ash. This was conducted based on an acceptable calibration range at Cu Kα 8.00–8.05 while the instrument was operated at 50 kV and 30 μA.
A Micromeritics' ASAP® 2020 Accelerated Surface Area and Porosity Analyser BET was used to analyse both fresh and spent K2SiO3. The procedures using the BET instrument were initiated with the samples being dried with nitrogen purging. The volume of the gas being adsorbed at the surface of the particles was measured by referring to the boiling point of nitrogen at −196 °C. The number of gas molecules adsorbed corresponds to the total particles on the surface area, which includes the pores present on the surface.
A FESEM-EDX, JEOL (JSM-7800f) with a spatial resolution up to 1 nm was used to study the surface morphology and elemental analysis of the fresh and spent K2SiO3.
The Hammett tests were carried out on spent and fresh catalyst of BA 900 and K2SiO3 where phenolphthalein, 2,4-dinitroaniline, 4-nitroaniline and methyl red were used as indicators to determine the qualitative acidic, as well as basic, properties of the catalyst. 25 mg of catalyst was weighed and prepared in three batches and 5 ml of methanol was added to the catalyst. Then, 1 ml of the indicator was added to 4 ml of methanol, with the final volume of 5 ml. The indicators were added separately to the catalysts, which were weighed in batches. The mixture was then left to equilibrate for 2 h and the colour changes were observed and noted.
Turn over frequency (TOF)
 | (1) |
Conclusions
In brief, boiler ash containing K2SiO3 (BA 900) and commercially available K2SiO3 both demonstrated high selectivity and yield toward glycerol carbonate formation with no loss of activity up to three consecutive cycles. Besides, it was proven that boiler ash containing potassium silicate can act as an attractive catalyst to selectively convert the polyols ethylene glycol and propylene glycol into their desired cyclic organic carbonates. Therefore, this material can serve as a Lewis acid catalyst for the synthesis of cyclic organic carbonates. In short, the catalyst derived from waste boiler ash can serve as an economical catalyst for the synthesis of glycerol carbonate, ethylene carbonate and propylene carbonate.Acknowledgements
The authors would like to thank the Universiti Malaysia Pahang, the Universiti Putra Malaysia and the Ministry of Higher Education for Research Acculturation Collaborative Effort grant (RACE, RDU121301) and the Universiti Malaysia Pahang for Internal Grants Scheme (RDU120363).References
- V. P. Indran, N. A. Syuhada Zuhaimi, M. A. Deraman, G. P. Maniam, M. M. Yusoff, T.-Y. Yun Hin and M. H. Ab. Rahim, RSC Adv., 2014, 4, 25257–25267 RSC.
- C. Barreto, E. Hansen and S. Fredriksen, Polym. Degrad. Stab., 2012, 97, 893–904 CrossRef CAS.
- E. Perricone, M. Chamas, L. Cointeaux, J.-C. Leprêtre, P. Judeinstein, P. Azais, F. Béguin and F. Alloin, Electrochim. Acta, 2013, 93, 1–7 CrossRef CAS.
- R. Wagner, S. Brox, J. Kasnatscheew, D. R. Gallus, M. Amereller, I. Cekic-Laskovic and M. Winter, Electrochem. Commun., 2014, 40, 80–83 CrossRef CAS.
- M. Aresta, A. Dibenedetto, F. Nocito and C. Pastore, J. Mol. Catal. A: Chem., 2006, 257, 149–153 CrossRef CAS.
- A. Dibenedetto, A. Angelini, M. Aresta, J. Ethiraj, C. Fragale and F. Nocito, Tetrahedron, 2011, 67, 1308–1313 CrossRef CAS.
- S. C. Kim, Y. H. Kim, H. Lee, D. Y. Yoon and B. K. Song, J. Mol. Catal. B: Enzym., 2007, 49, 75–78 CrossRef CAS.
- M. Aresta, A. Dibenedetto, F. Nocito and C. Ferragina, J. Catal., 2009, 268, 106–114 CrossRef CAS.
- S. Fujita, Y. Yamanishi and M. Arai, J. Catal., 2013, 297, 137–141 CrossRef CAS.
- N. A. S. Zuhaimi, V. P. Indran, M. A. Deraman, N. F. Mudrikah, G. P. Maniam, Y. H. Taufiq-Yap and M. H. Ab. Rahim, Appl. Catal., A, 2015, 502, 312–319 CrossRef CAS.
- Y. T. Algoufi and B. H. Hameed, Fuel Process. Technol., 2014, 126, 5–11 CrossRef CAS.
- S. Musić, N. Filipović-Vinceković and L. Sekovanić, Braz. J. Chem. Eng., 2011, 28, 89–94 Search PubMed.
- X. Zhao, H. An, S. Wang, F. Li and Y. Wang, J. Chem. Technol. Biotechnol., 2008, 83, 750–755 CrossRef CAS.
- P. Sharma, R. Dwivedi, R. Dixit and R. Prasad, Ind. Eng. Chem. Res., 2013, 53, 10977–10987 CrossRef.
- D. Wang, X. Zhang, T. Cheng, J. Wu and Q. Xue, AIMS Bioeng., 2014, 2, 399–409 Search PubMed.
- P. Qiu, X. D. Jiang, C. Kang and B. L. Yang, Adv. Mater. Res., 2012, 550–553, 141–144 CrossRef CAS.
- Z. Du, F. Chen, Z. Lin, X. Li, H. Yuan and Y. Wu, Chem. Eng. J., 2014, 278, 79–84 CrossRef.
- A. Lundström, T. Snelling, P. Morsing, P. Gabrielsson, E. Senar and L. Olsson, Appl. Catal., B, 2011, 106, 273–279 CrossRef.
- X. Zhao, Y. Zhang and Y. Wang, Ind. Eng. Chem. Res., 2004, 43, 4038–4042 CrossRef CAS.
- B. M. Bhanage, S. Fujita, Y. Ikushima and M. Arai, Green Chem., 2003, 5, 429 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: XRF of BA 110 as well as 13C NMR of ethylene carbonate and propylene carbonate. See DOI: 10.1039/c5ra26286k |
| This journal is © The Royal Society of Chemistry 2016 |