Amphoteric nanoporous polybenzimidazole membrane with extremely low crossover for a vanadium redox flow battery†
Abstract
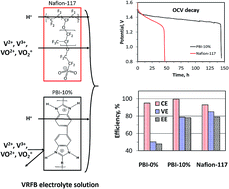
We report amphoteric polybenzimidazole (PBI) membranes with tailored nanoporous structures for vanadium redox flow batteries (VRFBs). The amphoteric nanoporous membrane has restricted the crossover of vanadium species and instantaneously allowed for the transport of protons owing to the positively charged polybenzimidazolium polymer backbone. The selected membrane shows a proton/vanadium (H/V) selectivity of about 133, which is considerably higher than those stated in previous reports. This reflects its high coulombic efficiency of 99.4%, energy efficiency of 78.2%, and low capacity decay rate of 0.27% per cycle when compared to 93.0%, 78.9%, and 0.58% per cycle of Nafion-117, respectively.

 Please wait while we load your content...
Please wait while we load your content...