Synthesis of 1H-azadienes and application to one-pot organic transformations†
Yearang Kwon,
Mina Jeon,
Jin Yong Park,
Young Ho Rhee* and
Jaiwook Park*
Department of Chemistry, POSTECH (Pohang University of Science and Technology), Pohang, 790-784, Korea. E-mail: pjw@postech.ac.kr; Web: http://oml.postech.ac.kr Fax: +82-54-279-0654
First published on 17th December 2015
Abstract
1H-Azadienes were synthesized from allyl azides by ruthenium catalysis under mild and neutral conditions. Applications of the 1H-azadienes were demonstrated for the one-pot synthesis of N-benzyl-1-azadienes, hydrazones, tertiary carbinamines, dieneamide, and N-cyclohexenylacetamides. Two 1H-azatrienes were also synthesized, which were transformed to pyridine derivatives.
α,β-Unsaturated imines are highly versatile building blocks for the synthesis of various nitrogen-containing compounds, because they act as N-nucleophiles1 as well as electrophiles in 1,2-addition and Michael-type 1,4-addition reactions.2 They also participate as heterodienes in cycloaddition reactions to give heterocyclic compounds. Including the classical condensation reaction between α,β-unsaturated carbonyl compounds and amines,2a,c,g there are many methods to afford α,β-unsaturated imines having various substituents on the nitrogen.3 However, those for N-unsubstituted α,β-unsaturated imines (1H-azadienes), which can be regarded as the synthetically most versatile type, are rare and limited in substrate scope.4–6 In fact, N-unsubstituted imines (N–H imines) are known to have low stability and utilized as in situ generated intermediates (Scheme 1). One of the most common methods to generate 1H-azadienes is the three component reaction of phosphonates, nitriles, and aldehydes.4 However, the substrate scope is limited to linear α,β-unsaturated N–H ketimines that can survive under conditions using organolithium reagents. Another method is the indium-mediated 1,2-addition reaction toward α,β-unsaturated nitriles.5 This method also has a limited substrate scope, because only simple allyl- and benzyl bromides are effective to give α,β-unsaturated N–H ketimines. An intermediate 1H-azadiene in the reaction of ammonia and an α,β-unsaturated carbonyl compound has been reported, which was subjected to the in situ reaction with allyl boronates to give a homoallylic amine. However, the use of excessive ammonia was required for the tandem reaction, and only the result of employing non-enolizable verbenone was reported.6 Recently, a variation of the condensation reaction using Ti(OiPr)4 and triethylamine as additives has been developed and applied for the synthesis of dienamides.7 The use of excessive Ti(OiPr)4 requires a complicated work-up procedure to remove titanium species from the reaction mixture. Herein we wish to report a new and simple method for the synthesis of a wide range of 1H-azadienes from allyl azides under mild and neutral conditions using a diruthenium catalyst 1. The 1H-azadienes were characterized by NMR and IR spectroscopy and utilized in one-pot transformations to give valuable nitrogen-containing compounds.
Recently, we found an interesting catalytic activity of the diruthenium complex 1 transforming alkyl azides to N–H imines under the illumination of household fluorescent light.8 Although the N–H imines produced from aliphatic azides are generally unstable due to isomerization and self-condensation reactions, those from benzylic azides are stable enough to be accumulated for the subsequent reactions such as the allylation with allylboranes,8a the Rh-catalyzed annulation reaction with alkynes to give isoquinolines8b and synthesis of enamides.8c Then we envisioned that the conjugation effect would facilitate the accumulation of 1H-azadienes generated by our catalyst system and that the synthesis of 1H-azadienes from ally azides will highly extend the utility of our catalyst system.
First, we examined the effect of well-known dynamic 1,3-rearrangement of allyl azides9 on the transformation to 1H-azadienes (Scheme 2). The transformation of a 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 mixture of the primary azide 2a and the secondary azide 2a′ gave the aldimine and the ketimine 3a′ with a ratio (36
53 mixture of the primary azide 2a and the secondary azide 2a′ gave the aldimine and the ketimine 3a′ with a ratio (36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64) similar to that of the starting azides in 95% combined yield. However, the aldimine 3b was obtained exclusively in 94% yield from an 80
64) similar to that of the starting azides in 95% combined yield. However, the aldimine 3b was obtained exclusively in 94% yield from an 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 mixture of the primary azide 2b and the tertiary azide 2b′. The transformation of the phenyl-conjugated allyl azide 2c also exclusively gave the aldimine 3c in 97% yield. The results of Scheme 2 implicate that the ratio of regioisomeric N–H imines are practically parallel with that of starting allyl azides except the case of tertiary allyl azides, which are unreactive toward the Ru-catalysis. Meanwhile, the E/Z ratios of the N–H group range from 7
20 mixture of the primary azide 2b and the tertiary azide 2b′. The transformation of the phenyl-conjugated allyl azide 2c also exclusively gave the aldimine 3c in 97% yield. The results of Scheme 2 implicate that the ratio of regioisomeric N–H imines are practically parallel with that of starting allyl azides except the case of tertiary allyl azides, which are unreactive toward the Ru-catalysis. Meanwhile, the E/Z ratios of the N–H group range from 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 5
3 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, which were estimated by the intensity of imine protons in the 1H NMR spectra.10
5, which were estimated by the intensity of imine protons in the 1H NMR spectra.10
In Chart 1, the results from the catalytic transformation of various allylic azides to 1H-azadienes are summarized, which show the substrate scope of functional group compatibility and structural variation. As shown in Scheme 2c, aryl-conjugated allyl azides were transformed selectively to the corresponding 4-aryl-1H-azadienes 3d–v. The yield of 3e was slightly lower among those of 3d–f having a methyl substituent on phenyl ring probably due to a steric effect of the ortho-methyl substituent. However, there is no clear trend for the electronic effect of aromatic groups: the yields of the electron-poor ones (3i and 3k) were lower than that of the electron-rich one (3l), while those of 4-fluorophenyl derivative (3h) and 4-bromophenyl derivative (3j) were comparable with that of 3l. It is notable that 3m, which has a ketone group, was obtained in good yield; this compound cannot be provided by the conventional methods involving the condensation reaction of carbonyl compounds with amines or the reduction of nitrile groups. Another interesting compound is 3n, which has an acid-labile formacetal group. Derivatives having naphthyl group (3o) and heteroaromatic rings (3p–r) were also obtained in quantitative yields. High yields of 3s and 3t showed that substituent variation at the iminyl carbon would be fairly extensible with our method. The methyl substituent on C-3 and C-4 position in 3u and 3v led to the formation of mixtures of geometric isomers with about 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E/Z selectivity. 2-Azidooct-3-ene and 4-azidooct-2-ene were in equilibrium to give a 57
1 E/Z selectivity. 2-Azidooct-3-ene and 4-azidooct-2-ene were in equilibrium to give a 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43 mixture, and the mixture was converted to the 54
43 mixture, and the mixture was converted to the 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 mixture of oct-3-en-2-imine (3w) and oct-2-en-4-imine (3w′). Cyclohex-2-enimine (3x), which cannot be afforded by the methods employing nitriles, was obtained in 77% yield. Compound 3y which has ester group was also successfully synthesized. Interestingly, 1-cyclohexenylethanimine (3z) was obtained selectively in 78% yield from a 3
46 mixture of oct-3-en-2-imine (3w) and oct-2-en-4-imine (3w′). Cyclohex-2-enimine (3x), which cannot be afforded by the methods employing nitriles, was obtained in 77% yield. Compound 3y which has ester group was also successfully synthesized. Interestingly, 1-cyclohexenylethanimine (3z) was obtained selectively in 78% yield from a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioisomeric mixture of 1-(1-azidoethyl)cyclohex-1-ene and 1-azido-2-ethylidenecyclohexane.11
1 regioisomeric mixture of 1-(1-azidoethyl)cyclohex-1-ene and 1-azido-2-ethylidenecyclohexane.11
Compound 3aa is an example of 1H-azatriene, which could be converted to a pyridine derivative (4a) through a one-pot oxidative cyclization (Scheme 3).12 The low yield of 4a is probably due to the (E)-configuration of the central carbon–carbon double bond, which should be isomerized for the cyclization. In fact, the yield for a bicyclic pyridine derivative (4b) was higher than that of 4a in the transformation of another 1H-azatriene (3ab) having a central carbon–carbon double bond of (Z)-configuration.
To demonstrate the distinct utility of our method, we selected the cyclic 1H-azadiene 3y containing an ester group, which cannot be afforded by the known methods employing nitrile substrates and/or strong nucleophiles, for the reactions with three nucleophiles and an electrophile (Scheme 4).
In comparison to the conventional condensation reaction between ketones and amines to give N-substituted imines, the transamination reaction of 3 with benzylamine was very fast and completed in 30 minutes at room temperature.2a,c,g,3b–d. The transimination reaction with phenylhydrazine was also facile. The nucleophilic allylation with allylboronate was highly selective to give tertiary carbinamines 7.2h,6,13 The reaction with acetic anhydride is an example showing the nucleophilicity of 1H-azadienes; the N-acetylation of 3y occurs first and isomerization to the corresponding dienamide 8 follows.8c In comparison with the recent synthesis of dienamides by the direct condensation of α,β-unsaturated ketones and ammonia in the presence of excess Ti(OiPr),7b our synthetic method has definite advantages in efficiency, particularly in the simple purification of the resulting products, not requiring the tricky removal of titanium species from the reaction mixture.
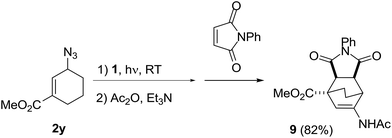
Furthermore, the dienamide 8 was a good substrate for the reaction with N-phenylmaleic imide to give the corresponding cycloaddition product 9,14 which would be an important building block for the synthesis of various natural products and medicinal compounds.15 The cycloaddition product 9 having an ester group was obtained in 82% isolated yields through a one-pot three-step reaction from the cyclic allyl azides 2y (Scheme 5). Noticeably, the cyclization reactions were highly stereoselective to give a single diastereoisomer; the ester group on the cyclohexene ring is trans to the cyclic imide ring.16
Conclusions
We synthesized a wide range of 1H-azadines, including those difficult to be prepared by conventional methods, from primary and secondary allyl azides by a Ru-catalysis under mild and neutral conditions. The 1H-azadines were characterized by IR and NMR spectroscopy, and utilized in one-step organic transformations to afford N-substituted 1-azadiens, tertiary carbinamines, N-acetyl dienamides, and N-cyclohexenylacetamides. In addition, by a parallel procedure, 1H-azatrienes were prepared and transformed to pyridine derivatives.Experimental
General information
Air-sensitive manipulations were carried out with standard Schlenk techniques under argon atmosphere. Commercial chemicals were used without further purification. Flash column chromatography was carried out on silica gel (230–400 mesh) as the stationary phase. 1H and 13C NMR spectra were recorded with a 300 MHz spectrometer. 1H NMR spectra were referenced to residual CDCl3 (7.26 ppm) or THF-d8 (3.58 ppm) and reported as follows; chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet). Chemical shift of 13C NMR spectra were measured relative to CDCl3 (77.23 ppm), THF-d8 (67.57 ppm). 2D NMR spectra (COSY, HSQC and NOESY) were recorded on a 600 MHz spectrometer. Infrared spectra were recorded for the samples in KBr pellets. Mass spectral data were obtained from the Korea Basic Science Institute (Daegu). The light source was a household fluorescent light (30 W; 220 V, 60 Hz).General procedure for synthesis of 1H-azadienes
In an NMR tube filled with argon gas the ruthenium catalyst 1 (1.0–4.0 mol%) and an azide 2 (0.25 mmol) were dissolved in dry THF-d8 (0.5 mL), and the resulting solution was irradiated with fluorescent light (30 W) for 1–2 h at ambient temperature. The yield of the corresponding 1H-azadienes was estimated by 1H NMR using nitromethane or dibromomethane as an internal standard. The 1H-azadiene 3d was analysed by 1D- and 2D-NMR techniques (COSY, NOESY, and HSQC), and the resonance peaks for its E and Z isomers were assigned. The ratio of E and Z isomers of other 1H-azadienes were estimated by 1H NMR on the basis of the assignment for 3d.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N-3a
(Z)-N-3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ((E)-N-3a′ + (Z)-N-3a′) = 24
((E)-N-3a′ + (Z)-N-3a′) = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64; 1H NMR (300 MHz): (E)-N-3a: δ = 10.10 (d, 1H, J = 16.0 Hz), 8.29 (dd, 1H, J = 8.4, 16.0 Hz), 6.42–6.33 (m, 1H), 6.28 (m, 1H), 4.34–4.29 (m, 2H), 0.90 (s, 9H), 0.06 (s, 6H); (Z)-N-3a: δ = 9.66 (d, 1H, J = 25 Hz), 7.88 (dd, 1H, J = 8.2, 25.0 Hz), 6.26–6.19 (m, 1H), 6.17–6.08 (m, 1H), 4.34–4.29 (m, 2H), 0.94 (s, 9H), 0.09 (s, 6H); (E)-N-3a′ + (Z)-N-3a′: δ = 10.17 (m, 1H) 6.45 (dd, 1H, J = 11.4, 18.4 Hz), 5.74 (d, 1H, J = 18.4), 5.50 (d, 1H, 11.4 Hz), 0.96 (s, 9H), 0.12 (s, 6H); 13C NMR (75 MHz): 3a + 3a′: δ = 173.2, 170.4, 169.3, 144.9, 142.5, 137.4, 133.4, 132.8, 132.3, 130.6, 128.9, 120.9, 63.6, 63.2, 63.1, 26.5, 26.4, 26.4, 19.2, 19.0, 18.9, 1.6, −2.5, −2.6, −4.9, −4.9, −5.1; IR (NaCl): ν = 3244, 3223, 1652, 1473, 1442, 1438 cm−1.
64; 1H NMR (300 MHz): (E)-N-3a: δ = 10.10 (d, 1H, J = 16.0 Hz), 8.29 (dd, 1H, J = 8.4, 16.0 Hz), 6.42–6.33 (m, 1H), 6.28 (m, 1H), 4.34–4.29 (m, 2H), 0.90 (s, 9H), 0.06 (s, 6H); (Z)-N-3a: δ = 9.66 (d, 1H, J = 25 Hz), 7.88 (dd, 1H, J = 8.2, 25.0 Hz), 6.26–6.19 (m, 1H), 6.17–6.08 (m, 1H), 4.34–4.29 (m, 2H), 0.94 (s, 9H), 0.09 (s, 6H); (E)-N-3a′ + (Z)-N-3a′: δ = 10.17 (m, 1H) 6.45 (dd, 1H, J = 11.4, 18.4 Hz), 5.74 (d, 1H, J = 18.4), 5.50 (d, 1H, 11.4 Hz), 0.96 (s, 9H), 0.12 (s, 6H); 13C NMR (75 MHz): 3a + 3a′: δ = 173.2, 170.4, 169.3, 144.9, 142.5, 137.4, 133.4, 132.8, 132.3, 130.6, 128.9, 120.9, 63.6, 63.2, 63.1, 26.5, 26.4, 26.4, 19.2, 19.0, 18.9, 1.6, −2.5, −2.6, −4.9, −4.9, −5.1; IR (NaCl): ν = 3244, 3223, 1652, 1473, 1442, 1438 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 65
(Z)-N = 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35; 1H NMR (300 MHz): (E)-N-3b: δ = 9.86 (d, 1H, J = 16.0 Hz), 9.83 (d, 1H, J = 16.0 Hz), 8.61 (dd, 1H, J = 9.1, 16.0 Hz), 8.58 (dd, 1H, J = 9.1, 16.0 Hz), 5.93 (dd, 1H, J = 1.1, 9.1 Hz), 5.17–5.06 (m, 1H), 2.22–2.01 (m, 4H), 1.93 (d, 3H, J = 1.1 Hz), 1.86 (d, 3H, J = 1.1 Hz): (Z)-N-3b: δ = 9.45 (d, 1H, J = 24.9 Hz), 9.40 (d, 1H, J = 24.9 Hz), 8.21 (dd, 1H, J = 8.9, 24.9 Hz), 8.17 (dd, 1H, J = 8.9, 24.9 Hz), 5.65 (dd, 1H, J = 1.1, 8.9 Hz), 1.89 (d, 3H, J = 1.1 Hz), 1.81 (d, 3H, J = 1.1 Hz); 13C NMR (75 MHz): (E)-N-3b + (Z)-N-3b: δ = 167.4, 167.1, 166.4, 166.0, 150.9, 150.7, 149.0, 148.9, 132.9, 132.7, 132.6, 129.6, 128.8, 128.6, 127.7, 124.7, 124.5, 124.5, 124.4, 41.1, 40.7, 33.5, 33.4, 28.0, 27.2, 27.1, 26.0, 24.2, 17.9, 17.2; IR (NaCl): ν = 3251, 3222, 3216, 1682, 1673, 1638, 1492, 1468, 1456 cm−1.
35; 1H NMR (300 MHz): (E)-N-3b: δ = 9.86 (d, 1H, J = 16.0 Hz), 9.83 (d, 1H, J = 16.0 Hz), 8.61 (dd, 1H, J = 9.1, 16.0 Hz), 8.58 (dd, 1H, J = 9.1, 16.0 Hz), 5.93 (dd, 1H, J = 1.1, 9.1 Hz), 5.17–5.06 (m, 1H), 2.22–2.01 (m, 4H), 1.93 (d, 3H, J = 1.1 Hz), 1.86 (d, 3H, J = 1.1 Hz): (Z)-N-3b: δ = 9.45 (d, 1H, J = 24.9 Hz), 9.40 (d, 1H, J = 24.9 Hz), 8.21 (dd, 1H, J = 8.9, 24.9 Hz), 8.17 (dd, 1H, J = 8.9, 24.9 Hz), 5.65 (dd, 1H, J = 1.1, 8.9 Hz), 1.89 (d, 3H, J = 1.1 Hz), 1.81 (d, 3H, J = 1.1 Hz); 13C NMR (75 MHz): (E)-N-3b + (Z)-N-3b: δ = 167.4, 167.1, 166.4, 166.0, 150.9, 150.7, 149.0, 148.9, 132.9, 132.7, 132.6, 129.6, 128.8, 128.6, 127.7, 124.7, 124.5, 124.5, 124.4, 41.1, 40.7, 33.5, 33.4, 28.0, 27.2, 27.1, 26.0, 24.2, 17.9, 17.2; IR (NaCl): ν = 3251, 3222, 3216, 1682, 1673, 1638, 1492, 1468, 1456 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 71
(Z)-N = 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29; 1H NMR (300 MHz): (E)-N-3c: δ = 10.27 (d, 1H, J = 15.9 Hz), 8.42 (dd, 1H, J = 8.2, 15.9 Hz), 7.65–7.48 (m, 2H), 7.39–7.22 (m, 3H), 7.02 (d, 1H, J = 16.1 Hz), 6.90 (ddd, 1H, J = 1.1, 8.2, 16.1 Hz); (Z)-N-3c: δ = 9.87 (d, 1H, J = 24.9), 8.04 (dd, 1H, J = 8.6, 24.9 Hz), 6.96 (d, 1H, J = 15.8 Hz), 6.62 (ddd, 1H, J = 0.8, 8.5, 15.8 Hz); 13C NMR (75 MHz): (E)-N-3c + (Z)-N-3c: δ = 171.0, 169.9, 143.2, 142.2, 140.5, 138.2, 136.9, 133.9, 131.7, 130.3, 130.2, 130.0, 129.8, 129.7, 129.7, 129.6, 129.3, 128.4, 128.3, 128.2, 128.1, 127.4; IR (NaCl): ν = 3261, 3221, 1675, 1632, 1496, 1449 cm−1.
29; 1H NMR (300 MHz): (E)-N-3c: δ = 10.27 (d, 1H, J = 15.9 Hz), 8.42 (dd, 1H, J = 8.2, 15.9 Hz), 7.65–7.48 (m, 2H), 7.39–7.22 (m, 3H), 7.02 (d, 1H, J = 16.1 Hz), 6.90 (ddd, 1H, J = 1.1, 8.2, 16.1 Hz); (Z)-N-3c: δ = 9.87 (d, 1H, J = 24.9), 8.04 (dd, 1H, J = 8.6, 24.9 Hz), 6.96 (d, 1H, J = 15.8 Hz), 6.62 (ddd, 1H, J = 0.8, 8.5, 15.8 Hz); 13C NMR (75 MHz): (E)-N-3c + (Z)-N-3c: δ = 171.0, 169.9, 143.2, 142.2, 140.5, 138.2, 136.9, 133.9, 131.7, 130.3, 130.2, 130.0, 129.8, 129.7, 129.7, 129.6, 129.3, 128.4, 128.3, 128.2, 128.1, 127.4; IR (NaCl): ν = 3261, 3221, 1675, 1632, 1496, 1449 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 55
(Z)-N = 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45; 1H NMR (300 MHz): (E)-N-3d: δ = 9.76 (s, 1H), 7.56–7.51 (m, 2H), 7.36–7.26 (m, 3H), 7.11 (d, 1H, J = 16.6 Hz), 6.93 (d, 1H, J = 16.6 Hz), 2.18 (s, 3H); (Z)-N-3d: δ = 9.63 (s, 1H), 6.94 (d, 1H, J = 16.3 Hz), 6.71 (d, 1H, J = 16.3), 2.21 (s, 3H); 13C NMR (75 MHz): (E)-N-3d + (Z)-N-3d: δ = 173.5, 173.4, 137.5, 137.4, 137.1, 135.8, 131.9, 130.2, 129.7, 129.6, 129.5, 129.5, 128.1, 128.1; IR (NaCl): ν = 3252, 1630, 1494, 1449 cm−1.
45; 1H NMR (300 MHz): (E)-N-3d: δ = 9.76 (s, 1H), 7.56–7.51 (m, 2H), 7.36–7.26 (m, 3H), 7.11 (d, 1H, J = 16.6 Hz), 6.93 (d, 1H, J = 16.6 Hz), 2.18 (s, 3H); (Z)-N-3d: δ = 9.63 (s, 1H), 6.94 (d, 1H, J = 16.3 Hz), 6.71 (d, 1H, J = 16.3), 2.21 (s, 3H); 13C NMR (75 MHz): (E)-N-3d + (Z)-N-3d: δ = 173.5, 173.4, 137.5, 137.4, 137.1, 135.8, 131.9, 130.2, 129.7, 129.6, 129.5, 129.5, 128.1, 128.1; IR (NaCl): ν = 3252, 1630, 1494, 1449 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 52
(Z)-N = 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48; 1H NMR (300 MHz): (E)-N-3e: δ = 9.72 (s, 1H), 7.60–7.49 (m, 1H), 7.20–7.11 (m, 3H), 7.33 (d, 1H, J = 16.6 Hz), 6.78 (d, 1H, J = 16.6 Hz), 2.38 (s, 3H), 2.18 (d, 3H, J = 1.41 Hz); (Z)-N-3e: δ = 9.60 (s, 1H), 7.15 (d, 1H, J = 16.3 Hz), 6.66 (dd, 1H, J = 0.71, 16.3 Hz), 2.20 (s, 3H); 13C NMR (75 MHz): (E)-N-3e + (Z)-N-3e: δ = 173.6, 173.5, 137.6, 137.2, 136.3, 135.9, 135.0, 133.4, 133.2, 131.6, 131.4, 131.3, 129.6, 129.4, 127.2, 127.2, 126.8, 126.6, 24.8, 22.2, 19.9, 19.9; IR (NaCl): ν = 3210, 1487, 1461 cm−1.
48; 1H NMR (300 MHz): (E)-N-3e: δ = 9.72 (s, 1H), 7.60–7.49 (m, 1H), 7.20–7.11 (m, 3H), 7.33 (d, 1H, J = 16.6 Hz), 6.78 (d, 1H, J = 16.6 Hz), 2.38 (s, 3H), 2.18 (d, 3H, J = 1.41 Hz); (Z)-N-3e: δ = 9.60 (s, 1H), 7.15 (d, 1H, J = 16.3 Hz), 6.66 (dd, 1H, J = 0.71, 16.3 Hz), 2.20 (s, 3H); 13C NMR (75 MHz): (E)-N-3e + (Z)-N-3e: δ = 173.6, 173.5, 137.6, 137.2, 136.3, 135.9, 135.0, 133.4, 133.2, 131.6, 131.4, 131.3, 129.6, 129.4, 127.2, 127.2, 126.8, 126.6, 24.8, 22.2, 19.9, 19.9; IR (NaCl): ν = 3210, 1487, 1461 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 52
(Z)-N = 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48; 1H NMR (300 MHz): (E)-N-3f: δ = 9.69 (s, 1H), 7.36–7.28 (m, 2H), 7.23–7.18 (m, 1H), 7.11–7.08 (m, 1H), 7.05 (d, 1H, J = 16.7 Hz), 6.87 (d, 1H, J = 16.7 Hz), 2.32 (s, 3H), 2.16 (d, 3H, J = 1.59 Hz); (Z)-N-3f: δ = 9.55 (s, 1H), 6.88 (d, 1H, J = 16.1 Hz), 6.66 (dd, 1H, J = 0.59, 16.1 Hz), 2.17 (s, 3H); 13C NMR (75 MHz): (E)-N-3f + (Z)-N-3f: δ = 173.5, 173.4, 139.2, 139.1, 137.7, 137.4, 137.1, 136.0, 131.8, 130.6, 130.3, 130.2, 129.5, 129.5, 128.9, 128.8, 125.5, 125.4, 24.8, 22.1, 21.5; IR (NaCl): ν = 3215, 1495, 1436 cm−1.
48; 1H NMR (300 MHz): (E)-N-3f: δ = 9.69 (s, 1H), 7.36–7.28 (m, 2H), 7.23–7.18 (m, 1H), 7.11–7.08 (m, 1H), 7.05 (d, 1H, J = 16.7 Hz), 6.87 (d, 1H, J = 16.7 Hz), 2.32 (s, 3H), 2.16 (d, 3H, J = 1.59 Hz); (Z)-N-3f: δ = 9.55 (s, 1H), 6.88 (d, 1H, J = 16.1 Hz), 6.66 (dd, 1H, J = 0.59, 16.1 Hz), 2.17 (s, 3H); 13C NMR (75 MHz): (E)-N-3f + (Z)-N-3f: δ = 173.5, 173.4, 139.2, 139.1, 137.7, 137.4, 137.1, 136.0, 131.8, 130.6, 130.3, 130.2, 129.5, 129.5, 128.9, 128.8, 125.5, 125.4, 24.8, 22.1, 21.5; IR (NaCl): ν = 3215, 1495, 1436 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 54
(Z)-N = 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46; 1H NMR (300 MHz): (E)-N-3g: δ = 9.65 (s, 1H), 7.43–7.38 (m, 2H), 7.16–7.13 (m, 2H), 7.04 (d, 1H, J = 16.6 Hz), 6.84 (d, 1H, J = 16.6 Hz), 2.31 (s, 3H), 2.15 (d, 3H, J = 1.54 Hz); (Z)-N-3g: δ = 9.51 (s, 1H), 6.88 (d, 1H, J = 16.2 Hz), 6.62 (dd, 1H, J = 0.58, 16.2 Hz), 2.16 (s, 3H); 13C NMR (75 MHz): (E)-N-3g + (Z)-N-3g: δ = 173.5, 173.4, 139.9, 139.5, 137.5, 135.8, 134.8, 134.4, 131.1, 130.3, 130.3, 129.5, 128.2, 128.1, 24.8, 22.1, 21.5; IR (NaCl): ν = 3211, 1440, 1403 cm−1.
46; 1H NMR (300 MHz): (E)-N-3g: δ = 9.65 (s, 1H), 7.43–7.38 (m, 2H), 7.16–7.13 (m, 2H), 7.04 (d, 1H, J = 16.6 Hz), 6.84 (d, 1H, J = 16.6 Hz), 2.31 (s, 3H), 2.15 (d, 3H, J = 1.54 Hz); (Z)-N-3g: δ = 9.51 (s, 1H), 6.88 (d, 1H, J = 16.2 Hz), 6.62 (dd, 1H, J = 0.58, 16.2 Hz), 2.16 (s, 3H); 13C NMR (75 MHz): (E)-N-3g + (Z)-N-3g: δ = 173.5, 173.4, 139.9, 139.5, 137.5, 135.8, 134.8, 134.4, 131.1, 130.3, 130.3, 129.5, 128.2, 128.1, 24.8, 22.1, 21.5; IR (NaCl): ν = 3211, 1440, 1403 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 57
(Z)-N = 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43; 1H NMR (300 MHz): (E)-N-3h: δ = 9.72 (s, 1H), 7.60–7.52 (m, 2H), 7.11–7.05 (m, 2H), 7.07 (d, 1H, J = 16.5 Hz), 6.84 (d, 1H, J = 16.5 Hz), 2.16 (d, 3H, J = 1.35 Hz); (Z)-N-3h: δ = 9.57 (s, 1H), 6.91 (d, 1H, J = 16.2 Hz), 6.63 (d, 1H, J = 16.2 Hz), 2.17 (s, 3H); 13C NMR (75 MHz): (E)-N-3h + (Z)-N-3h: δ = 173.4, 173.3, 164.2 (d, J = 247 Hz), 164.0 (d, J = 248 Hz), 136.3, 134.6, 133.9 (d, J = 3 Hz), 133.6 (d, J = 3 Hz), 131.9 (d, J = 2 Hz), 130.3 (d, J = 2 Hz), 130.2, 130.1, 116.7 (d, J = 6 Hz), 116.4 (d, J = 6 Hz), 24.7, 22.1; IR (NaCl): ν = 3210, 1508, 1439 cm−1.
43; 1H NMR (300 MHz): (E)-N-3h: δ = 9.72 (s, 1H), 7.60–7.52 (m, 2H), 7.11–7.05 (m, 2H), 7.07 (d, 1H, J = 16.5 Hz), 6.84 (d, 1H, J = 16.5 Hz), 2.16 (d, 3H, J = 1.35 Hz); (Z)-N-3h: δ = 9.57 (s, 1H), 6.91 (d, 1H, J = 16.2 Hz), 6.63 (d, 1H, J = 16.2 Hz), 2.17 (s, 3H); 13C NMR (75 MHz): (E)-N-3h + (Z)-N-3h: δ = 173.4, 173.3, 164.2 (d, J = 247 Hz), 164.0 (d, J = 248 Hz), 136.3, 134.6, 133.9 (d, J = 3 Hz), 133.6 (d, J = 3 Hz), 131.9 (d, J = 2 Hz), 130.3 (d, J = 2 Hz), 130.2, 130.1, 116.7 (d, J = 6 Hz), 116.4 (d, J = 6 Hz), 24.7, 22.1; IR (NaCl): ν = 3210, 1508, 1439 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 57
(Z)-N = 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43; 1H NMR (300 MHz): (E)-N-3i: δ = 9.78 (s, 1H), 7.54–7.49 (m, 2H), 7.36–7.34 (m, 2H), 7.06 (d, 1H, J = 16.7 Hz), 6.89 (d, 1H, J = 16.7 Hz), 2.16 (d, 3H, J = 1.31 Hz); (Z)-N-3i: δ = 9.62 (s, 1H), 6.90 (d, 1H, J = 16.4 Hz), 6.68 (d, 1H, J = 16.4 Hz), 2.17 (s, 3H); 13C NMR (75 MHz): (E)-N-3i + (Z)-N-3i: δ = 172.3, 172.2, 135.1, 135.0, 134.8, 134.2, 133.9, 133.3, 131.6, 129.8, 128.7, 128.7, 128.5, 128.5, 128.3, 128.3, 127.9, 23.5, 21.0; IR (NaCl): ν = 3210, 1548, 1491 cm−1.
43; 1H NMR (300 MHz): (E)-N-3i: δ = 9.78 (s, 1H), 7.54–7.49 (m, 2H), 7.36–7.34 (m, 2H), 7.06 (d, 1H, J = 16.7 Hz), 6.89 (d, 1H, J = 16.7 Hz), 2.16 (d, 3H, J = 1.31 Hz); (Z)-N-3i: δ = 9.62 (s, 1H), 6.90 (d, 1H, J = 16.4 Hz), 6.68 (d, 1H, J = 16.4 Hz), 2.17 (s, 3H); 13C NMR (75 MHz): (E)-N-3i + (Z)-N-3i: δ = 172.3, 172.2, 135.1, 135.0, 134.8, 134.2, 133.9, 133.3, 131.6, 129.8, 128.7, 128.7, 128.5, 128.5, 128.3, 128.3, 127.9, 23.5, 21.0; IR (NaCl): ν = 3210, 1548, 1491 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 58
(Z)-N = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42; 1H NMR (300 MHz): (E)-N-3j: δ = 9.68 (s, 1H), 7.41–7.30 (m, 4H), 6.94 (d, 1H, J = 16.6 Hz), 6.78 (d, 1H, J = 16.6 Hz), 2.06 (s, 3H); (Z)-N-3j: δ = 9.51 (s, 1H), 7.41–7.30 (m, 4H), 6.79 (d, 1H, J = 16.2 Hz), 6.58 (d, 1H, J = 16.2 Hz), 2.05 (s, 3H); 13C NMR (75 MHz): (E)-N-3j + (Z)-N-3j: δ = 173.3, 173.2, 136.6, 136.3, 136.1, 132.8, 132.7, 132.7, 130.9, 129.8, 123.5, 123.2, 24.6, 22.0; IR (NaCl): ν = 3208, 1594, 1584, 1486, 1439 cm−1.
42; 1H NMR (300 MHz): (E)-N-3j: δ = 9.68 (s, 1H), 7.41–7.30 (m, 4H), 6.94 (d, 1H, J = 16.6 Hz), 6.78 (d, 1H, J = 16.6 Hz), 2.06 (s, 3H); (Z)-N-3j: δ = 9.51 (s, 1H), 7.41–7.30 (m, 4H), 6.79 (d, 1H, J = 16.2 Hz), 6.58 (d, 1H, J = 16.2 Hz), 2.05 (s, 3H); 13C NMR (75 MHz): (E)-N-3j + (Z)-N-3j: δ = 173.3, 173.2, 136.6, 136.3, 136.1, 132.8, 132.7, 132.7, 130.9, 129.8, 123.5, 123.2, 24.6, 22.0; IR (NaCl): ν = 3208, 1594, 1584, 1486, 1439 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 59
(Z)-N = 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41; 1H (300 MHz): (E)-N-3k: δ = 9.88 (s, 1H), 8.01–7.98 (m, 2H), 7.66–7.60 (m, 2H), 7.15 (d, 1H, J = 16.6 Hz), 7.00 (d, 1H, J = 16.6 Hz), 3.85 (s, 3H), 2.19 (d, 3H, J = 1.56); (Z)-N-3k: δ = 9.72 (s, 1H), 6.98 (d, 1H, J = 16.3 Hz), 6.80 (d, 1H, J = 16.3 Hz), 2.20 (s, 3H); 13C NMR (75 MHz): (E)-N-3k + (Z)-N-3k: δ = 173.4, 173.3, 172.7, 142.2, 141.9, 141.6, 139.3, 136.4, 134.6, 134.4, 133.9, 132.4, 131.5, 131.2, 130.8, 128.2, 128.1, 127.2, 52.3, 52.3, 24.7, 22.1; IR (NaCl): ν = 3247, 3220, 1720, 1435, 1413 cm−1.
41; 1H (300 MHz): (E)-N-3k: δ = 9.88 (s, 1H), 8.01–7.98 (m, 2H), 7.66–7.60 (m, 2H), 7.15 (d, 1H, J = 16.6 Hz), 7.00 (d, 1H, J = 16.6 Hz), 3.85 (s, 3H), 2.19 (d, 3H, J = 1.56); (Z)-N-3k: δ = 9.72 (s, 1H), 6.98 (d, 1H, J = 16.3 Hz), 6.80 (d, 1H, J = 16.3 Hz), 2.20 (s, 3H); 13C NMR (75 MHz): (E)-N-3k + (Z)-N-3k: δ = 173.4, 173.3, 172.7, 142.2, 141.9, 141.6, 139.3, 136.4, 134.6, 134.4, 133.9, 132.4, 131.5, 131.2, 130.8, 128.2, 128.1, 127.2, 52.3, 52.3, 24.7, 22.1; IR (NaCl): ν = 3247, 3220, 1720, 1435, 1413 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 54
(Z)-N = 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46; 1H NMR (300 MHz): (E)-N-3l: δ = 9.56 (s, 1H), 7.48–7.42 (m, 2H), 7.05–6.79 (m, 2H), 7.02 (d, 1H, J = 16.6 Hz), 6.84 (d, 1H, J = 16.6 Hz), 3.77 (s, 3H), 2.15 (s, 3H); (Z)-N-3l: δ = 9.57 (s, 1H), 6.86 (d, 1H, J = 16.2 Hz), 6.54 (d, 1H, J = 16.2 Hz); 13C NMR (75 MHz): (E)-N-3l + (Z)-N-3l: δ = 173.5, 161.7, 161.5, 137.2, 135.6, 130.0, 129.8, 129.6, 129.5, 128.2, 115.1, 115.1, 55.6, 24.8, 22.1; IR (NaCl): ν = 3214, 1486, 1461 cm−1.
46; 1H NMR (300 MHz): (E)-N-3l: δ = 9.56 (s, 1H), 7.48–7.42 (m, 2H), 7.05–6.79 (m, 2H), 7.02 (d, 1H, J = 16.6 Hz), 6.84 (d, 1H, J = 16.6 Hz), 3.77 (s, 3H), 2.15 (s, 3H); (Z)-N-3l: δ = 9.57 (s, 1H), 6.86 (d, 1H, J = 16.2 Hz), 6.54 (d, 1H, J = 16.2 Hz); 13C NMR (75 MHz): (E)-N-3l + (Z)-N-3l: δ = 173.5, 161.7, 161.5, 137.2, 135.6, 130.0, 129.8, 129.6, 129.5, 128.2, 115.1, 115.1, 55.6, 24.8, 22.1; IR (NaCl): ν = 3214, 1486, 1461 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 56
(Z)-N = 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44; 1H NMR (300 MHz): (E)-N-3m: δ = 9.81 (s, 1H), 8.13–8.12 (m, 1H), 7.92–7.88 (m, 1H), 7.79–7.71 (m, 1H), 7.49–7.42 (m, 1H), 7.18 (d, 1H, J = 16.7 Hz), 6.97 (d, 1H, J = 16.7 Hz), 2.57 (s, 3H), 2.20 (s, 3H); (Z)-N-3m: δ = 9.67 (s, 1H), 7.01 (d, 1H, J = 16.3 Hz), 6.79 (dd, 1H, J = 0.68, 16.3 Hz), 2.56 (s, 3H), 2.19 (s, 3H); 13C NMR (75 MHz): (E)-N-3m + (Z)-N-3m: δ = 197.1, 197.0, 173.4, 173.3, 139.0, 138.0, 136.7, 135.0, 133.1, 132.1, 131.9, 131.3, 129.1, 129.9, 129.5, 129.2, 128.2, 128.0, 26.7, 26.7, 24.7, 22.1; IR (NaCl): ν = 3249, 1684, 1633, 1595 cm−1.
44; 1H NMR (300 MHz): (E)-N-3m: δ = 9.81 (s, 1H), 8.13–8.12 (m, 1H), 7.92–7.88 (m, 1H), 7.79–7.71 (m, 1H), 7.49–7.42 (m, 1H), 7.18 (d, 1H, J = 16.7 Hz), 6.97 (d, 1H, J = 16.7 Hz), 2.57 (s, 3H), 2.20 (s, 3H); (Z)-N-3m: δ = 9.67 (s, 1H), 7.01 (d, 1H, J = 16.3 Hz), 6.79 (dd, 1H, J = 0.68, 16.3 Hz), 2.56 (s, 3H), 2.19 (s, 3H); 13C NMR (75 MHz): (E)-N-3m + (Z)-N-3m: δ = 197.1, 197.0, 173.4, 173.3, 139.0, 138.0, 136.7, 135.0, 133.1, 132.1, 131.9, 131.3, 129.1, 129.9, 129.5, 129.2, 128.2, 128.0, 26.7, 26.7, 24.7, 22.1; IR (NaCl): ν = 3249, 1684, 1633, 1595 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 53
(Z)-N = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47; 1H NMR (300 MHz): (E)-N-3n: δ = 9.50 (s, 1H), 7.00 (d, 1H, J = 16.7 Hz), 6.84 (s, 1H), 6.75–6.66 (m, 2H), 5.85 (s, 2H), 2.05 (s, 3H); (Z)-N-3n: δ = 9.35 (s, 1H), 6.89 (d, 1H, J = 16.2 Hz), 6.83 (s, 1H), 6.42 (d, 1H, J = 16.2 Hz), 2.03 (s, 3H); 13C NMR (75 MHz): (E)-N-3n + (Z)-N-3n: δ = 173.7, 149.9, 137.6, 135.9, 132.2, 131.8, 130.5, 129.2, 128.9, 124.2, 123.9, 109.4, 107.1, 102.9, 25.1, 22.4; IR (NaCl): ν = 3244, 3218, 1630, 1602, 1491, 1484 cm−1.
47; 1H NMR (300 MHz): (E)-N-3n: δ = 9.50 (s, 1H), 7.00 (d, 1H, J = 16.7 Hz), 6.84 (s, 1H), 6.75–6.66 (m, 2H), 5.85 (s, 2H), 2.05 (s, 3H); (Z)-N-3n: δ = 9.35 (s, 1H), 6.89 (d, 1H, J = 16.2 Hz), 6.83 (s, 1H), 6.42 (d, 1H, J = 16.2 Hz), 2.03 (s, 3H); 13C NMR (75 MHz): (E)-N-3n + (Z)-N-3n: δ = 173.7, 149.9, 137.6, 135.9, 132.2, 131.8, 130.5, 129.2, 128.9, 124.2, 123.9, 109.4, 107.1, 102.9, 25.1, 22.4; IR (NaCl): ν = 3244, 3218, 1630, 1602, 1491, 1484 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 56
(Z)-N = 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44; 1H NMR (300 MHz): (E)-N-3o: δ = 9.64 (s, 1H), 8.92 (s, 1H), 7.81–7.56 (m, 5H), 7.36–7.26 (m, 2H), 7.14 (d, 1H, J = 16.7 Hz), 6.91 (d, 1H, J = 16.7 Hz), 2.09 (s, 3H), 1.74 (s, 3H); (Z)-N-3o: δ = 9.51 (s, 1H), 8.84 (s, 1H), 6.97 (d, 1H, J = 16.4 Hz), 6.69 (d, 1H, J = 16.4 Hz), 2.11 (s, 3H), 1.78 (s, 3H); 13C NMR (75 MHz): (E)-N-3o + (Z)-N-3o: δ = 173.5, 173.4, 137.5, 135.9, 134.9, 134.7, 134.6, 132.3, 130.5, 129.3, 129.2, 129.1, 128.9, 128.5, 128.4, 127.4, 127.3, 127.2, 125.8, 124.5, 124.3, 22.1, 24.7; IR (NaCl): ν = 3210, 1627, 1599, 1589, 1488, 1435 cm−1.
44; 1H NMR (300 MHz): (E)-N-3o: δ = 9.64 (s, 1H), 8.92 (s, 1H), 7.81–7.56 (m, 5H), 7.36–7.26 (m, 2H), 7.14 (d, 1H, J = 16.7 Hz), 6.91 (d, 1H, J = 16.7 Hz), 2.09 (s, 3H), 1.74 (s, 3H); (Z)-N-3o: δ = 9.51 (s, 1H), 8.84 (s, 1H), 6.97 (d, 1H, J = 16.4 Hz), 6.69 (d, 1H, J = 16.4 Hz), 2.11 (s, 3H), 1.78 (s, 3H); 13C NMR (75 MHz): (E)-N-3o + (Z)-N-3o: δ = 173.5, 173.4, 137.5, 135.9, 134.9, 134.7, 134.6, 132.3, 130.5, 129.3, 129.2, 129.1, 128.9, 128.5, 128.4, 127.4, 127.3, 127.2, 125.8, 124.5, 124.3, 22.1, 24.7; IR (NaCl): ν = 3210, 1627, 1599, 1589, 1488, 1435 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 53
(Z)-N = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47; 1H NMR (300 MHz): (E)-N-3p: δ = 9.66 (s, 1H), 7.39–7.35 (m, 1H), 7.25 (d, 1H, J = 16.4 Hz), 7.19–7.17 (m, 1H), 6.65 (d, 1H, J = 16.3 Hz), 7.02–6.98 (m, 1H), 2.13 (d, 3H, J = 1.54 Hz); (Z)-N-3p: δ = 9.57 (s, 1H), 7.08 (d, 1H, J = 16.0 Hz), 6.46 (d, 1H, J = 16.0 Hz), 2.14 (s, 3H); 13C NMR (75 MHz): (E)-N-3p + (Z)-N-3p: δ = 173.1, 173.0142.6, 142.2, 131.2, 130.4, 129.8, 129.4, 129.2, 128.8, 128.8, 128.7, 127.6, 127.3, 24.7, 22.0; IR (NaCl): ν = 3211, 1437, 1428 cm−1.
47; 1H NMR (300 MHz): (E)-N-3p: δ = 9.66 (s, 1H), 7.39–7.35 (m, 1H), 7.25 (d, 1H, J = 16.4 Hz), 7.19–7.17 (m, 1H), 6.65 (d, 1H, J = 16.3 Hz), 7.02–6.98 (m, 1H), 2.13 (d, 3H, J = 1.54 Hz); (Z)-N-3p: δ = 9.57 (s, 1H), 7.08 (d, 1H, J = 16.0 Hz), 6.46 (d, 1H, J = 16.0 Hz), 2.14 (s, 3H); 13C NMR (75 MHz): (E)-N-3p + (Z)-N-3p: δ = 173.1, 173.0142.6, 142.2, 131.2, 130.4, 129.8, 129.4, 129.2, 128.8, 128.8, 128.7, 127.6, 127.3, 24.7, 22.0; IR (NaCl): ν = 3211, 1437, 1428 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 53
(Z)-N = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47; 1H NMR (300 MHz): (E)-N-3q: δ = 9.67 (s, 1H), 7.53 (s, 1H), 6.91 (d, 1H, J = 16.5 Hz), 6.71 (d, 1H, J = 16.5 Hz), 6.54–6.43 (m, 2H), 2.11 (d, 3H, J = 1.53 Hz); (Z)-N-3q: δ = 9.58 (s, 1H), 6.73 (d, 1H, J = 16.1 Hz), 6.48 (d, 1H, J = 15.9 Hz) 2.13 (s, 3H); 13C NMR (75 MHz): (E)-N-3q + (Z)-N-3q: δ = 173.1, 173.1, 153.5, 153.0, 144.9, 144.6, 129.9, 128.0, 124.9, 123.3, 112.9, 112.9, 112.8, 112.0, 24.7, 21.9; IR (NaCl): ν = 3209, 1497, 1479 cm−1.
47; 1H NMR (300 MHz): (E)-N-3q: δ = 9.67 (s, 1H), 7.53 (s, 1H), 6.91 (d, 1H, J = 16.5 Hz), 6.71 (d, 1H, J = 16.5 Hz), 6.54–6.43 (m, 2H), 2.11 (d, 3H, J = 1.53 Hz); (Z)-N-3q: δ = 9.58 (s, 1H), 6.73 (d, 1H, J = 16.1 Hz), 6.48 (d, 1H, J = 15.9 Hz) 2.13 (s, 3H); 13C NMR (75 MHz): (E)-N-3q + (Z)-N-3q: δ = 173.1, 173.1, 153.5, 153.0, 144.9, 144.6, 129.9, 128.0, 124.9, 123.3, 112.9, 112.9, 112.8, 112.0, 24.7, 21.9; IR (NaCl): ν = 3209, 1497, 1479 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 50
(Z)-N = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; 1H NMR (300 MHz): (E)-N-3r: δ = 9.90 (s, 1H), 8.55 (d, 1H, J = 4.59 Hz), 7.71–7.65 (m, 1H), 7.46 (dd, 1H, J = 7.84, 21.2 Hz), 7.32 (d, 1H, J = 16.5 Hz), 7.21–7.13 (m, 1H), 7.12 (d, 1H, J = 16.5 Hz), 2.19 (d, 3H, J = 1.59); (Z)-N-3r: δ = 9.78 (s, 1H), 6.93 (d, 1H, J = 16.0 Hz), 2.20 (s, 3H); 13C NMR (75 MHz): (E)-N-3r + (Z)-N-3r: δ = 173.5, 173.4, 156.0, 155.6, 150.9, 150.8, 137.4, 137.2, 135.4, 135.2, 133.6, 133.4, 124.2, 124.1, 123.8, 123.4, 24.9, 22.2; IR (NaCl): ν = 3221, 1431, 1404 cm−1.
50; 1H NMR (300 MHz): (E)-N-3r: δ = 9.90 (s, 1H), 8.55 (d, 1H, J = 4.59 Hz), 7.71–7.65 (m, 1H), 7.46 (dd, 1H, J = 7.84, 21.2 Hz), 7.32 (d, 1H, J = 16.5 Hz), 7.21–7.13 (m, 1H), 7.12 (d, 1H, J = 16.5 Hz), 2.19 (d, 3H, J = 1.59); (Z)-N-3r: δ = 9.78 (s, 1H), 6.93 (d, 1H, J = 16.0 Hz), 2.20 (s, 3H); 13C NMR (75 MHz): (E)-N-3r + (Z)-N-3r: δ = 173.5, 173.4, 156.0, 155.6, 150.9, 150.8, 137.4, 137.2, 135.4, 135.2, 133.6, 133.4, 124.2, 124.1, 123.8, 123.4, 24.9, 22.2; IR (NaCl): ν = 3221, 1431, 1404 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 50
(Z)-N = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; 1H NMR (300 MHz): (E)-N-3s: δ = 10.0 (s, 1H), 7.74–7.70 (m, 1H), 7.43–7.38 (m, 2H), 7.33–7.23 (m, 4H), 7.21–7.13 (m, 3H), 7.10 (d, 1H, J = 16.3 Hz), 6.98 (d, 1H, J = 16.3 Hz); (Z)-N-3s: δ = 9.87 (s, 1H), 7.06 (d, 1H, J = 16.2 Hz), 6.88 (d, 1H, 16.2 Hz); 13C NMR (75 MHz): (E)-N-3s + (Z)-N-3s: δ = 176.1, 173.6, 141.7, 140.3, 140.0, 137.7, 137.2, 137.1, 130.1, 129.8, 129.7, 129.6, 129.6, 129.4, 129.0, 128.9, 128.3, 128.2, 127.7; IR (NaCl): ν = 3252, 3213, 1636, 1627, 1495, 1449 cm−1.
50; 1H NMR (300 MHz): (E)-N-3s: δ = 10.0 (s, 1H), 7.74–7.70 (m, 1H), 7.43–7.38 (m, 2H), 7.33–7.23 (m, 4H), 7.21–7.13 (m, 3H), 7.10 (d, 1H, J = 16.3 Hz), 6.98 (d, 1H, J = 16.3 Hz); (Z)-N-3s: δ = 9.87 (s, 1H), 7.06 (d, 1H, J = 16.2 Hz), 6.88 (d, 1H, 16.2 Hz); 13C NMR (75 MHz): (E)-N-3s + (Z)-N-3s: δ = 176.1, 173.6, 141.7, 140.3, 140.0, 137.7, 137.2, 137.1, 130.1, 129.8, 129.7, 129.6, 129.6, 129.4, 129.0, 128.9, 128.3, 128.2, 127.7; IR (NaCl): ν = 3252, 3213, 1636, 1627, 1495, 1449 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 51
(Z)-N = 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49; 1H NMR (300 MHz): (E)-N-3t; δ = 9.70 (s, 1H), 7.54–7.49 (m, 2H), 7.30–7.34 (m, 2H), 7.19 (d, 1H, J = 16.7 Hz), 6.87 (d, 1H, J = 16.7 Hz), 2.54 (t, 2H, J = 7.5 Hz), 1.65–1.72 (m, 2H), 1.45–1.35 (m, 2H), 0.95 (t, 3H, J = 7.4 Hz); (Z)-N-3t: δ = 9.66 (s, 1H), 6.96 (d, 1H, J = 16.4 Hz), 6.65 (d, 1H, J = 16.4), 2.47 (t, 2H, J = 7.77 Hz), 1.51–1.58 (m, 2H); 13C NMR (75 MHz): (E)-N-3t + (Z)-N-3t: δ = 176.7, 176.1, 137.5, 137.2, 136.4, 134.8, 131.1, 129.8, 129.6, 129.5, 129.4, 128.1, 128.0, 37.4, 35.4, 29.6, 29.3, 23.5, 23.3, 14.5, 14.4; IR (NaCl): ν = 3216, 1630, 1590, 1495, 1449 cm−1.
49; 1H NMR (300 MHz): (E)-N-3t; δ = 9.70 (s, 1H), 7.54–7.49 (m, 2H), 7.30–7.34 (m, 2H), 7.19 (d, 1H, J = 16.7 Hz), 6.87 (d, 1H, J = 16.7 Hz), 2.54 (t, 2H, J = 7.5 Hz), 1.65–1.72 (m, 2H), 1.45–1.35 (m, 2H), 0.95 (t, 3H, J = 7.4 Hz); (Z)-N-3t: δ = 9.66 (s, 1H), 6.96 (d, 1H, J = 16.4 Hz), 6.65 (d, 1H, J = 16.4), 2.47 (t, 2H, J = 7.77 Hz), 1.51–1.58 (m, 2H); 13C NMR (75 MHz): (E)-N-3t + (Z)-N-3t: δ = 176.7, 176.1, 137.5, 137.2, 136.4, 134.8, 131.1, 129.8, 129.6, 129.5, 129.4, 128.1, 128.0, 37.4, 35.4, 29.6, 29.3, 23.5, 23.3, 14.5, 14.4; IR (NaCl): ν = 3216, 1630, 1590, 1495, 1449 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 76
(Z)-N = 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24; 1H NMR (300 MHz): (E)-N-3u δ = 9.78 (s, 1H), 7.38–7.20 (m, 5H), 7.20–7.19 (m, 1H), 7.10–7.04 (m, 1H), 2.26 (s, 3H), 2.13 (s, 3H); (Z)-N-3u: δ = 9.69 (s, 1H), 7.38–7.20 (m, 5H), 7.20–7.19 (m, 1H), 7.10–7.04 (m, 1H), 2.26 (s, 3H), 2.13 (s 3H); 13C NMR (75 MHz): 3u + 3u′: δ = 175.3, 139.8, 138.7, 134.1, 133.3, 130.4, 130.3, 129.3, 129.2, 129.1, 129.0, 129.0, 128.3, 128.1, 127.9, 126.3, 26.0, 23.5, 23.4, 14.5, 13.7; IR (NaCl): ν = 3207, 1634, 1599 cm−1.
24; 1H NMR (300 MHz): (E)-N-3u δ = 9.78 (s, 1H), 7.38–7.20 (m, 5H), 7.20–7.19 (m, 1H), 7.10–7.04 (m, 1H), 2.26 (s, 3H), 2.13 (s, 3H); (Z)-N-3u: δ = 9.69 (s, 1H), 7.38–7.20 (m, 5H), 7.20–7.19 (m, 1H), 7.10–7.04 (m, 1H), 2.26 (s, 3H), 2.13 (s 3H); 13C NMR (75 MHz): 3u + 3u′: δ = 175.3, 139.8, 138.7, 134.1, 133.3, 130.4, 130.3, 129.3, 129.2, 129.1, 129.0, 129.0, 128.3, 128.1, 127.9, 126.3, 26.0, 23.5, 23.4, 14.5, 13.7; IR (NaCl): ν = 3207, 1634, 1599 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 33
(Z)-N = 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67; 1H NMR (300 MHz): (E)-N-3u′: δ = 9.57 (s, 1H), 7.38–7.20 (m, 5H), 6.24 (s, 1H), 2.12 (d, 3H, J = 1.36 Hz), 1.99 (s, 3H); (Z)-N-3u′: δ = 9.48 (s, 1H), 7.38–7.20 (m, 5H), 6.00 (s, 1H), 2.04 (s, 3H), 1.97 (s, 3H); 13C NMR (75 MHz): 3u + 3u′: δ = 175.3, 139.8, 138.7, 134.1, 133.3, 130.4, 130.3, 129.3, 129.2, 129.1, 129.0, 129.0, 128.3, 128.1, 127.9, 126.3, 26.0, 23.5, 23.4, 14.5, 13.7; IR (NaCl): ν = 3207, 1634, 1599 cm−1.
67; 1H NMR (300 MHz): (E)-N-3u′: δ = 9.57 (s, 1H), 7.38–7.20 (m, 5H), 6.24 (s, 1H), 2.12 (d, 3H, J = 1.36 Hz), 1.99 (s, 3H); (Z)-N-3u′: δ = 9.48 (s, 1H), 7.38–7.20 (m, 5H), 6.00 (s, 1H), 2.04 (s, 3H), 1.97 (s, 3H); 13C NMR (75 MHz): 3u + 3u′: δ = 175.3, 139.8, 138.7, 134.1, 133.3, 130.4, 130.3, 129.3, 129.2, 129.1, 129.0, 129.0, 128.3, 128.1, 127.9, 126.3, 26.0, 23.5, 23.4, 14.5, 13.7; IR (NaCl): ν = 3207, 1634, 1599 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 60
(Z)-N = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40; 1H (300 MHz): (E)-N-3v: δ = 9.75 (s, 1H), 7.58–7.04 (m, 5H), 6.27 (s, 1H), 2.50 (s, 3H), 2.09 (s, 3H); (Z)-N-3v: δ = 9.27 (s, 1H), 7.58–7.04 (m, 5H), 6.30 (s, 1H), 2.49 (s, 3H), 2.08 (s, 3H); 13C NMR (75 MHz): 3v + 3v′: δ = 175.4, 174.2, 147.6, 145.8, 145.1, 144.4, 143.6, 140.9, 129.5, 129.4, 129.3, 129.2, 129.1, 128.9, 128.7, 128.6, 128.4, 128.0, 127.9, 127.1, 126.9, 126.8, 109.5, 97.2, 31.1, 27.0, 26.8, 26.2, 23.3, 19.7, 18.3; IR (NaCl): ν = 3229, 1441, 1404 cm−1.
40; 1H (300 MHz): (E)-N-3v: δ = 9.75 (s, 1H), 7.58–7.04 (m, 5H), 6.27 (s, 1H), 2.50 (s, 3H), 2.09 (s, 3H); (Z)-N-3v: δ = 9.27 (s, 1H), 7.58–7.04 (m, 5H), 6.30 (s, 1H), 2.49 (s, 3H), 2.08 (s, 3H); 13C NMR (75 MHz): 3v + 3v′: δ = 175.4, 174.2, 147.6, 145.8, 145.1, 144.4, 143.6, 140.9, 129.5, 129.4, 129.3, 129.2, 129.1, 128.9, 128.7, 128.6, 128.4, 128.0, 127.9, 127.1, 126.9, 126.8, 109.5, 97.2, 31.1, 27.0, 26.8, 26.2, 23.3, 19.7, 18.3; IR (NaCl): ν = 3229, 1441, 1404 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 45
(Z)-N = 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55; 1H (300 MHz): (E)-N-3v′: δ = 9.44 (s, 1H), 7.58–7.04 (m, 5H), 6.00 (s, 1H), 2.18 (s, 3H), 2.07 (s, 3H); (Z)-N-3v: δ = 9.07 (s, 1H), 7.58–7.04 (m, 5H), 6.27 (s, 1H), 2.13 (s, 3H), 1.84 (s, 3H); 13C NMR (75 MHz): 3v + 3v′: δ = 175.4, 174.2, 147.6, 145.8, 145.1, 144.4, 143.6, 140.9, 129.5, 129.4, 129.3, 129.2, 129.1, 128.9, 128.7, 128.6, 128.4, 128.0, 127.9, 127.1, 126.9, 126.8, 109.5, 97.2, 31.1, 27.0, 26.8, 26.2, 23.3, 19.7, 18.3; IR (NaCl): ν = 3229, 1441, 1404 cm−1.
55; 1H (300 MHz): (E)-N-3v′: δ = 9.44 (s, 1H), 7.58–7.04 (m, 5H), 6.00 (s, 1H), 2.18 (s, 3H), 2.07 (s, 3H); (Z)-N-3v: δ = 9.07 (s, 1H), 7.58–7.04 (m, 5H), 6.27 (s, 1H), 2.13 (s, 3H), 1.84 (s, 3H); 13C NMR (75 MHz): 3v + 3v′: δ = 175.4, 174.2, 147.6, 145.8, 145.1, 144.4, 143.6, 140.9, 129.5, 129.4, 129.3, 129.2, 129.1, 128.9, 128.7, 128.6, 128.4, 128.0, 127.9, 127.1, 126.9, 126.8, 109.5, 97.2, 31.1, 27.0, 26.8, 26.2, 23.3, 19.7, 18.3; IR (NaCl): ν = 3229, 1441, 1404 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 56
(Z)-N = 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44; 1H NMR (300 MHz): (E)-N-3w: δ = 9.11 (s, 1H), 6.31–5.97 (m, 1H), 5.88–5.81 (m, 1H), 2.14–2.03 (m, 2H), 1.94 (s, 3H), 1.45–1.22 (m, 4H), 0.84 (t, 3H, J = 7.2 Hz); (Z)-N-3w: δ = 9.14 (s, 1H), 6.31–5.97 (m, 1H), 5.88–5.81 (m, 1H), 2.14–2.03 (m, 2H), 1.94 (s, 3H), 1.45–1.22 (m, 4H), 0.84 (t, 3H, J = 7.2 Hz); 13C NMR (75 MHz): 3w + 3w′: δ = 177.4, 176.1, 173.3, 140.3, 138.9, 135.1, 134.5, 134.3, 134.0, 133.5, 132.6, 132.4, 115.9, 37.2, 35.3, 33.4, 33.1, 32.2, 32.0, 29.6, 29.4, 25.9, 24.7, 23.6, 23.5, 23.3, 22.1, 19.6, 18.6, 18.3, 14.5, 14.4; IR (NaCl): ν = 3208, 1645, 1601, 1466, 1441 cm−1.
44; 1H NMR (300 MHz): (E)-N-3w: δ = 9.11 (s, 1H), 6.31–5.97 (m, 1H), 5.88–5.81 (m, 1H), 2.14–2.03 (m, 2H), 1.94 (s, 3H), 1.45–1.22 (m, 4H), 0.84 (t, 3H, J = 7.2 Hz); (Z)-N-3w: δ = 9.14 (s, 1H), 6.31–5.97 (m, 1H), 5.88–5.81 (m, 1H), 2.14–2.03 (m, 2H), 1.94 (s, 3H), 1.45–1.22 (m, 4H), 0.84 (t, 3H, J = 7.2 Hz); 13C NMR (75 MHz): 3w + 3w′: δ = 177.4, 176.1, 173.3, 140.3, 138.9, 135.1, 134.5, 134.3, 134.0, 133.5, 132.6, 132.4, 115.9, 37.2, 35.3, 33.4, 33.1, 32.2, 32.0, 29.6, 29.4, 25.9, 24.7, 23.6, 23.5, 23.3, 22.1, 19.6, 18.6, 18.3, 14.5, 14.4; IR (NaCl): ν = 3208, 1645, 1601, 1466, 1441 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 51
(Z)-N = 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49; 1H NMR (300 MHz): (E)-N-3w′: δ = 9.29 (s, 1H), 6.31–5.97 (m, 2H), 2.31–2.21 (m, 2H), 1.70 (dd, 3H, J = 1.3, 6.6 Hz) 1.45–1.22 (m, 4H), 0.84 (t, 3H, J = 7.2 Hz); (Z)-N-3w′: δ = 9.32 (s, 1H), 6.31–5.97 (m, 2H), 2.31–2.21 (m, 2H), 1.74 (dd, 3H, J = 1.2, 6.5 Hz), 1.45–1.22 (m, 4H), 0.84 (t, 3H, J = 7.2 Hz); 13C NMR (75 MHz): 3w + 3w′: δ = 177.4, 176.1, 173.3, 140.3, 138.9, 135.1, 134.5, 134.3, 134.0, 133.5, 132.6, 132.4, 115.9, 37.2, 35.3, 33.4, 33.1, 32.2, 32.0, 29.6, 29.4, 25.9, 24.7, 23.6, 23.5, 23.3, 22.1, 19.6, 18.6, 18.3, 14.5, 14.4; IR (NaCl): ν = 3208, 1645, 1601, 1466, 1441 cm−1.
49; 1H NMR (300 MHz): (E)-N-3w′: δ = 9.29 (s, 1H), 6.31–5.97 (m, 2H), 2.31–2.21 (m, 2H), 1.70 (dd, 3H, J = 1.3, 6.6 Hz) 1.45–1.22 (m, 4H), 0.84 (t, 3H, J = 7.2 Hz); (Z)-N-3w′: δ = 9.32 (s, 1H), 6.31–5.97 (m, 2H), 2.31–2.21 (m, 2H), 1.74 (dd, 3H, J = 1.2, 6.5 Hz), 1.45–1.22 (m, 4H), 0.84 (t, 3H, J = 7.2 Hz); 13C NMR (75 MHz): 3w + 3w′: δ = 177.4, 176.1, 173.3, 140.3, 138.9, 135.1, 134.5, 134.3, 134.0, 133.5, 132.6, 132.4, 115.9, 37.2, 35.3, 33.4, 33.1, 32.2, 32.0, 29.6, 29.4, 25.9, 24.7, 23.6, 23.5, 23.3, 22.1, 19.6, 18.6, 18.3, 14.5, 14.4; IR (NaCl): ν = 3208, 1645, 1601, 1466, 1441 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 50
(Z)-N = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50; 1H NMR (300 MHz): (E)-N-3x: δ = 9.01 (s, 1H), 6.28 (dtd, 1H, J = 1.5, 4.0, 10.1 Hz), 6.02 (d, 1H, J = 10.1 Hz), 2.2 (td, 1H, J = 1.5, 6.6 Hz), 2.09–2.01 (m, 2H), 1.75–1.64 (m, 2H); (Z)-N-3x: δ = 8.92 (s, 1H), 6.13 (td, 1H, J = 4.0, 9.9 Hz), 5.79 (d, 1H, J = 10.0 Hz), 2.30 (td, 1H, J = 1.2, 6.4 Hz); 13C NMR (75 MHz): (E)-N-3x + (Z)-N-3x: δ = 174.1, 173.9, 140.6, 140.0, 131.9, 130.6, 37.9, 35.1, 26.6, 26.5, 24.1, 24.1; IR (NaCl): ν = 3219, 1711, 1654, 1632, 1605, 1449, 1432 cm−1.
50; 1H NMR (300 MHz): (E)-N-3x: δ = 9.01 (s, 1H), 6.28 (dtd, 1H, J = 1.5, 4.0, 10.1 Hz), 6.02 (d, 1H, J = 10.1 Hz), 2.2 (td, 1H, J = 1.5, 6.6 Hz), 2.09–2.01 (m, 2H), 1.75–1.64 (m, 2H); (Z)-N-3x: δ = 8.92 (s, 1H), 6.13 (td, 1H, J = 4.0, 9.9 Hz), 5.79 (d, 1H, J = 10.0 Hz), 2.30 (td, 1H, J = 1.2, 6.4 Hz); 13C NMR (75 MHz): (E)-N-3x + (Z)-N-3x: δ = 174.1, 173.9, 140.6, 140.0, 131.9, 130.6, 37.9, 35.1, 26.6, 26.5, 24.1, 24.1; IR (NaCl): ν = 3219, 1711, 1654, 1632, 1605, 1449, 1432 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 53
(Z)-N = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47; 1H NMR (300 MHz): (E)-N-3y: δ = 9.88 (s, 1H), 7.01 (s, 1H), 3.73 (s, 3H), 2.46–4.33 (m, 4H), 1.87–1.77 (m, 2H); (Z)-N-3y: δ = 9.83 (s, 1H), 6.81 (s, 1H), 3.72 (s, 1H); 13C NMR (75 MHz): (E)-N-3y + (E)-N-3y: δ = 174.1, 173.6, 167.8, 167.6, 141.1, 139.5, 137.6, 134.8, 132.9, 128.6, 52.2, 52.2, 36.6, 34.1, 23.4, 23.3; IR(NaCl): ν = 3056, 2951, 1937, 1717, 1602, 1497, 1436, 1257, 1082 cm−1.
47; 1H NMR (300 MHz): (E)-N-3y: δ = 9.88 (s, 1H), 7.01 (s, 1H), 3.73 (s, 3H), 2.46–4.33 (m, 4H), 1.87–1.77 (m, 2H); (Z)-N-3y: δ = 9.83 (s, 1H), 6.81 (s, 1H), 3.72 (s, 1H); 13C NMR (75 MHz): (E)-N-3y + (E)-N-3y: δ = 174.1, 173.6, 167.8, 167.6, 141.1, 139.5, 137.6, 134.8, 132.9, 128.6, 52.2, 52.2, 36.6, 34.1, 23.4, 23.3; IR(NaCl): ν = 3056, 2951, 1937, 1717, 1602, 1497, 1436, 1257, 1082 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 68
(Z)-N = 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32; 1H NMR (300 MHz): (E)-N-3z: δ = 9.24 (s, 1H), 6.23–6.20 (m, 1H), 2.25–2.15 (m, 2H), 2.10–2.01 (m, 2H), 1.96 (s, 3H), 1.62–1.50 (m, 4H); (Z)-N-3z: δ = 9.18 (s, 1H), 1.96 (s, 3H); 13C NMR (75 MHz): (E)-N-3z + (Z)-N-3z: δ = 174.4, 173.8, 132.5, 132.3, 26.8, 26.5, 24.8, 24.1, 23.6, 23.4, 23.1, 22.9; IR(NaCl): ν = 3185, 3168, 1625, 1602, 1497, 1430 cm−1.
32; 1H NMR (300 MHz): (E)-N-3z: δ = 9.24 (s, 1H), 6.23–6.20 (m, 1H), 2.25–2.15 (m, 2H), 2.10–2.01 (m, 2H), 1.96 (s, 3H), 1.62–1.50 (m, 4H); (Z)-N-3z: δ = 9.18 (s, 1H), 1.96 (s, 3H); 13C NMR (75 MHz): (E)-N-3z + (Z)-N-3z: δ = 174.4, 173.8, 132.5, 132.3, 26.8, 26.5, 24.8, 24.1, 23.6, 23.4, 23.1, 22.9; IR(NaCl): ν = 3185, 3168, 1625, 1602, 1497, 1430 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Z)-N = 55
(Z)-N = 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45; 1H NMR (300 MHz): (E)-N-3aa: δ = 9.53 (s, 1H), 7.40–7.34 (m, 2H), 7.21–7.14 (m, 3H), 6.88–6.62 (m, 2H), 6.31 (d, 1H, J = 15.3 Hz), 1.99 (s, 3H); (Z)-N-3aa: δ = 9.36 (s, 1H), 6.05 (d, 1H, J = 15.1 Hz); 13C NMR (75 MHz): (E)-N-3aa + (Z)-N-3aa: δ = 173.3, 138.0, 136.9, 136.3, 135.8, 134.6, 133.6, 133.1, 129.5, 129.4, 129.3, 128.9, 128.6, 128.5, 127.6, 127.3, 24.7, 22.0; IR (NaCl): ν = 3214, 1611, 1584, 1572, 1496, 1448, 1398, 1349 cm−1.
45; 1H NMR (300 MHz): (E)-N-3aa: δ = 9.53 (s, 1H), 7.40–7.34 (m, 2H), 7.21–7.14 (m, 3H), 6.88–6.62 (m, 2H), 6.31 (d, 1H, J = 15.3 Hz), 1.99 (s, 3H); (Z)-N-3aa: δ = 9.36 (s, 1H), 6.05 (d, 1H, J = 15.1 Hz); 13C NMR (75 MHz): (E)-N-3aa + (Z)-N-3aa: δ = 173.3, 138.0, 136.9, 136.3, 135.8, 134.6, 133.6, 133.1, 129.5, 129.4, 129.3, 128.9, 128.6, 128.5, 127.6, 127.3, 24.7, 22.0; IR (NaCl): ν = 3214, 1611, 1584, 1572, 1496, 1448, 1398, 1349 cm−1.Oxidative cyclization of 1H-azatriene
A solution of 1 (4.9 mg, 2.0 mol%) and azide 2 (0.25 mmol) in THF (1.0 mL) was stirred for 2 h with illumination of 30 W fluorescent light at ambient temperature. Then reaction mixture was heated up to 100 °C under O2 atmosphere and stirred for 24 h. Then the solvent was removed under reduced pressure and the residue was purified by silica-gel column chromatography (n-hexane/ethyl acetate mixture). The azatriene 4 was obtained as yellow oil after drying under high vacuum.Synthesis of the imine 5
A solution of 2y (38 mg, 0.25 mmol), 1 (4.9 mg, 2.0 mol%) and benzyl amine (32 mg, 1.2 equiv.) in THF (1.0 mL) was stirred for 2 h with illumination of 30 W fluorescent light at ambient temperature. Then the solvent was removed under reduced pressure, and the yield (85%) of 5 was estimated by 1H NMR using CH2Br2 as an internal standard. 1H NMR (300 MHz, CDCl3): δ = 7.35–7.24 (m, 5H), 7.19 (s, 1H), 4.64 (s, 2H), 3.79 (s, 3H), 2.54–2.47 (m, 4H), 1.95–1.89 (m, 2H); 13C NMR (75 MHz, CDCl3): δ = 167.8, 167.1, 141.8, 139.3, 138.8, 129.3, 129.1, 129.0, 128.7, 128.6, 128.5, 127.9, 127.2, 127.1, 124.5, 56.0, 52.4, 52.1, 47.4, 27.2, 25.2, 22.8; IR (NaCl): ν = 3062, 3028, 2949, 2866, 1715, 1606, 1583, 1499, 1453, 1266, 1086 cm−1.Synthesis of the hydrazine 6
A solution of 2y (38 mg, 0.25 mmol) and 1 (4.9 mg, 2.0 mol%) in THF (1.0 mL) was stirred for 2 h with illumination of 30 W fluorescent light at ambient temperature. Then phenylhydrazine (32 mg, 1.2 equiv.), 1.0 M hydrogen chloride solution in ether (500 μL, 2.0 equiv.) and MeOH (1.0 mL) were added, and the resulting solution was stirred for 3 h at 70 °C. After concentration of crude mixture, salt was filtered through Celite. The residue was purified by silica-gel column chromatography (n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 10
ethyl acetate = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) afforded product 6 as yellow liquid (50 mg, 82%). 1H NMR (300 MHz, CDCl3): δ = 7.59 (s, 1H), 7.32–7.25 (m, 3H), 7.13–7.10 (m, 2H), 6.93–6.88 (t, 1H, J = 15.3 Hz), 3.78 (s, 3H), 2.48–2.37 (m, 4H), 1.95–1.89 (m, 2H); 13C NMR (75 MHz, CDCl3): δ = 168.0, 144.1, 142.6, 136.7, 130.9, 129.5, 121.1, 113.4, 51.9, 23.8, 22.3, 21.0; IR(NaCl): ν = 3323, 2949, 2027, 1704, 1601, 1556, 1507, 1436, 1234, 1195 cm−1; HRMS (FAB): m/z calculated for C14H16N2O2 [M]: 244.1212; found: 244.1216.
1) afforded product 6 as yellow liquid (50 mg, 82%). 1H NMR (300 MHz, CDCl3): δ = 7.59 (s, 1H), 7.32–7.25 (m, 3H), 7.13–7.10 (m, 2H), 6.93–6.88 (t, 1H, J = 15.3 Hz), 3.78 (s, 3H), 2.48–2.37 (m, 4H), 1.95–1.89 (m, 2H); 13C NMR (75 MHz, CDCl3): δ = 168.0, 144.1, 142.6, 136.7, 130.9, 129.5, 121.1, 113.4, 51.9, 23.8, 22.3, 21.0; IR(NaCl): ν = 3323, 2949, 2027, 1704, 1601, 1556, 1507, 1436, 1234, 1195 cm−1; HRMS (FAB): m/z calculated for C14H16N2O2 [M]: 244.1212; found: 244.1216.
Synthesis of the amine 7
A Solution of 2y (38 mg, 0.25 mmol), 1 (4.9 mg, 2.0 mol%) and allylboronic acid pinacol ester (130 mg, 3.0 equiv.) in THF (1.0 mL) was stirred for 3 h with illumination of 30 W fluorescent light at ambient temperature. The resulting mixture was extracted with aqueous 1 N HCl solution (2 × 2 mL) and basified with aqueous 6 N NaOH solution. The solution was extracted with Et2O (3 × 5 mL), dried over Na2SO4, filtered and concentrated under reduced pressure. The residue was purified by silica-gel column chromatography deactivated by Et3N using suitable eluent (CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 20
MeOH = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Product 7 was obtained as yellow liquid (41 mg, 84%) after drying under high vacuum. 1H NMR (300 MHz, CDCl3): δ = 6.71 (s, 1H), 5.88–5.79 (m, 1H), 5.18–5.12 (m, 2H), 3.73 (s, 3H), 2.27–2.17 (m, 4H), 1.74–1.64 (m, 3H), 1.54–1.49 (m, 1H), 1.43 (s, 2H); 13C NMR (75 MHz, CDCl3): δ = 168.2, 145.1, 133.2, 129.6, 119.3, 77.6, 77.2, 76.8, 51.8, 51.0, 46.6, 35.7, 24.5, 19.1; IR (NaCl): ν = 3360, 3292, 3075, 2938, 1716, 1641, 1590, 1436, 1247, 1087 cm−1; HRMS (EI) m/z calculated for C11H17NO2 [M + H]+: 196.1259; found: 196.1336.
1). Product 7 was obtained as yellow liquid (41 mg, 84%) after drying under high vacuum. 1H NMR (300 MHz, CDCl3): δ = 6.71 (s, 1H), 5.88–5.79 (m, 1H), 5.18–5.12 (m, 2H), 3.73 (s, 3H), 2.27–2.17 (m, 4H), 1.74–1.64 (m, 3H), 1.54–1.49 (m, 1H), 1.43 (s, 2H); 13C NMR (75 MHz, CDCl3): δ = 168.2, 145.1, 133.2, 129.6, 119.3, 77.6, 77.2, 76.8, 51.8, 51.0, 46.6, 35.7, 24.5, 19.1; IR (NaCl): ν = 3360, 3292, 3075, 2938, 1716, 1641, 1590, 1436, 1247, 1087 cm−1; HRMS (EI) m/z calculated for C11H17NO2 [M + H]+: 196.1259; found: 196.1336.
Synthesis of the dienamide 8
A solution of 2y (38 mg, 0.25 mmol) and 1 (4.9 mg, 2.0 mol%) in THF (1.0 mL) was stirred for 2 h with illumination of 30 W fluorescent light at ambient temperature. Then acetic anhydride (51 mg, 2.0 equiv.) and triethylamine (50 mg, 2.0 equiv.) were added, and the resulting solution was stirred for 12 h at 70 °C. Then the solvent was removed under reduced pressure and the residue was purified by silica-gel column chromatography (n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 1
ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Product 8 was obtained as white solid (41 mg, 83%) after drying under high vacuum. Mp 103–105 °C; 1H NMR (300 MHz, CDCl3): δ = 7.13 (s, 1H), 6.97 (d, 1H, J = 1.25), 6.31–6.29 (m, 1H), 3.76 (s, 3H), 2.47–2.41 (m, 2H), 2.36–2.28 (m, 2H), 2.07 (s, 3H); 13C NMR (75 MHz, CDCl3): δ = 174.45, 169.0, 167.5, 132.4, 131.8, 129.1, 120.4, 117.3, 51.9, 24.2, 22.1, 20.9; IR (NaCl): ν = 3292, 3185, 3055, 2925, 2046, 1997, 1714, 1549, 1436, 1373, 1263, 1196 cm−1; HRMS (FAB) m/z calculated for C10H13NO3 [M]: 195.0895; found: 195.0894.
1). Product 8 was obtained as white solid (41 mg, 83%) after drying under high vacuum. Mp 103–105 °C; 1H NMR (300 MHz, CDCl3): δ = 7.13 (s, 1H), 6.97 (d, 1H, J = 1.25), 6.31–6.29 (m, 1H), 3.76 (s, 3H), 2.47–2.41 (m, 2H), 2.36–2.28 (m, 2H), 2.07 (s, 3H); 13C NMR (75 MHz, CDCl3): δ = 174.45, 169.0, 167.5, 132.4, 131.8, 129.1, 120.4, 117.3, 51.9, 24.2, 22.1, 20.9; IR (NaCl): ν = 3292, 3185, 3055, 2925, 2046, 1997, 1714, 1549, 1436, 1373, 1263, 1196 cm−1; HRMS (FAB) m/z calculated for C10H13NO3 [M]: 195.0895; found: 195.0894.
Synthesis of the N-cyclohexenylacetamide 9
A solution of 2y (38 mg, 0.25 mmol) and 1 (4.9 mg, 2.0 mol%) in THF (1.0 mL) was stirred for 2 h with illumination of 30 W fluorescent light at ambient temperature. Then acetic anhydride (51 mg, 2.0 equiv.) and triethylamine (50 mg, 2.0 equiv.) were added, and the resulting solution was stirred for 6 h at 70 °C. N-Phenylmaleimide (86 mg, 2.0 equiv.) was added, and the resulting solution was stirred for 6 h at 70 °C. Then the solvent was removed under reduced pressure and the residue was purified by silica-gel column chromatography (n-hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate = 1
ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3). Product 9 was obtained as white solid (76 mg, 82%) after drying under high vacuum. Mp 128–130 °C; 1H NMR (300 MHz, CDCl3): δ = 7.44–7.32 (m, 3H), 7.11–7.08 (m, 2H), 6.92 (br s, 1H), 6.86 (s, 1H), 3.84 (s, 3H), 3.56–3.53 (m, 1H), 3.14–3.11 (m, 2H), 2.04–1.98 (m, 1H), 1.95 (s, 3H), 1.79–1.61 (m, 3H); 13C NMR (75 MHz, CDCl3): δ = 177.1, 175.8, 173.1, 168.7, 136.5, 131.5, 129.2, 128.8, 126.5, 109.0, 52.6, 47.3, 46.6, 44.0, 36.7, 29.8, 23.8, 16.5; IR (NaCl): ν = 3355, 3176, 3065, 2952, 2253, 1711, 1598, 1538, 1436, 1242, 1189, 1067 cm−1; HRMS (FAB): m/z calculated for C20H20N2O5 [M + H]+: 369.1372; found: 369.1450.
3). Product 9 was obtained as white solid (76 mg, 82%) after drying under high vacuum. Mp 128–130 °C; 1H NMR (300 MHz, CDCl3): δ = 7.44–7.32 (m, 3H), 7.11–7.08 (m, 2H), 6.92 (br s, 1H), 6.86 (s, 1H), 3.84 (s, 3H), 3.56–3.53 (m, 1H), 3.14–3.11 (m, 2H), 2.04–1.98 (m, 1H), 1.95 (s, 3H), 1.79–1.61 (m, 3H); 13C NMR (75 MHz, CDCl3): δ = 177.1, 175.8, 173.1, 168.7, 136.5, 131.5, 129.2, 128.8, 126.5, 109.0, 52.6, 47.3, 46.6, 44.0, 36.7, 29.8, 23.8, 16.5; IR (NaCl): ν = 3355, 3176, 3065, 2952, 2253, 1711, 1598, 1538, 1436, 1242, 1189, 1067 cm−1; HRMS (FAB): m/z calculated for C20H20N2O5 [M + H]+: 369.1372; found: 369.1450.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2015R1A2A2A01008130).Notes and references
- (a) D. A. Colby, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2008, 130, 3645 CrossRef CAS PubMed; (b) B. Groenendaal, E. Ruijter and R. V. A. Orru, Chem. Commun., 2008, 5474 RSC; (c) K. M. Oberg and T. Rovis, J. Am. Chem. Soc., 2011, 133, 4785 CrossRef CAS PubMed; (d) J.-L. Li, T.-Y. Liu and Y.-C. Chen, Acc. Chem. Res., 2012, 45, 1491 CrossRef CAS PubMed; (e) X. Jiang and R. Wang, Chem. Rev., 2013, 113, 5515 CrossRef CAS PubMed; (f) G. Masson, C. Lalli, M. Benohoud and G. Dagousset, Chem. Soc. Rev., 2013, 42, 902 RSC.
- (a) J. P. McMahon and J. A. Ellman, Org. Lett., 2005, 7, 5393 CrossRef CAS PubMed; (b) M. Shimizu, I. Hachiya and I. Mizota, Chem. Commun., 2009, 874 RSC; (c) C. Sole and E. Fernández, Chem.–Asian J., 2009, 4, 1790 CAS; (d) J. Liu and J. Hu, Chem.–Eur. J., 2010, 16, 11443 CrossRef CAS PubMed; (e) V. Pace, L. Castoldi, P. Hoyos, J. V. Sinisterra, M. Pregnolato and J. M. Sánchez-Montero, Tetrahedron, 2011, 67, 2670 CrossRef CAS; (f) F. Zhang, Z.-J. Liu and J.-T. Liu, Org. Biomol. Chem., 2011, 9, 3625 RSC; (g) A. D. J. Calow, A. S. Batsanov, E. Fernandez, C. Sole and A. Whiting, Chem. Commun., 2012, 48, 11401 RSC; (h) D. L. Silverio, S. Torker, T. Pilyugina, E. M. Vieira, M. L. Snapper, F. Haeffner and A. H. Hoveyda, Nature, 2013, 494, 216 CrossRef CAS PubMed.
- (a) K. Uneyama and H. Watanabe, Tetrahedron Lett., 1991, 32, 1459 CrossRef CAS; (b) L. Blackburn and R. J. K. Taylor, Org. Lett., 2001, 3, 1637 CrossRef CAS PubMed; (c) J. W. Rigoli, S. A. Moyer, S. D. Pearce and J. M. Schomaker, Org. Biomol. Chem., 2012, 10, 1746 RSC; (d) S. Morales, F. G. Guijarro, J. L. García Ruano and M. B. Cid, J. Am. Chem. Soc., 2013, 136, 1082 CrossRef PubMed.
- (a) A. S. Kiselyov, Tetrahedron Lett., 1995, 36, 9297 CrossRef CAS; (b) D. J. Vugts, H. Jansen, R. F. Schmitz, F. J. J. de Kanter and R. V. A. Orru, Chem. Commun., 2003, 2594 RSC; (c) F. Palacios, A. M. Ochoa de Retana, S. Pascual and J. Oyarzabal, J. Org. Chem., 2004, 69, 8767 CrossRef CAS PubMed; (d) A. S. Kiselyov, Tetrahedron Lett., 2005, 46, 1663 CrossRef CAS; (e) A. S. Kiselyov and L. Smith Ii, Tetrahedron Lett., 2006, 47, 2611 CrossRef CAS; (f) A. S. Kiselyov, Tetrahedron Lett., 2006, 47, 2941 CrossRef CAS; (g) M. Paravidino, R. S. Bon, R. Scheffelaar, D. J. Vugts, A. Znabet, R. F. Schmitz, F. J. J. de Kanter, M. Lutz, A. L. Spek, M. B. Groen and R. V. A. Orru, Org. Lett., 2006, 8, 5369 CrossRef CAS PubMed; (h) D. J. Vugts, M. M. Koningstein, R. F. Schmitz, F. J. J. de Kanter, M. B. Groen and R. V. A. Orru, Chem.–Eur. J., 2006, 12, 7178 CrossRef CAS PubMed; (i) F. Palacios, A. M. Ochoa de Retana, S. Pascual, G. F. de Trocóniz and J. M. Ezpeleta, Eur. J. Org. Chem., 2010, 6618 CrossRef CAS; (j) R. Scheffelaar, M. Paravidino, A. Znabet, R. F. Schmitz, F. J. J. de Kanter, M. Lutz, A. L. Spek, C. F. Guerra, F. M. Bickelhaupt, M. B. Groen, E. Ruijter and R. V. A. Orru, J. Org. Chem., 2010, 75, 1723 CrossRef CAS PubMed; (k) D. Coffinier, L. E. Kaim, L. Grimaud, E. Ruijter and R. V. A. Orru, Tetrahedron Lett., 2011, 52, 3023 CrossRef CAS; (l) F. Palacios, A. M. Ochoa de Retana, S. Pascual and G. Fernández de Trocóniz, Tetrahedron, 2011, 67, 1575 CrossRef CAS; (m) A. Kruithof, M. L. Ploeger, E. Janssen, M. Helliwell, F. J. J. D. Kanter, E. Ruijter and R. V. A. Orru, Molecules, 2012, 17, 1675 CrossRef CAS PubMed.
- B. C. Ranu and A. Das, Tetrahedron Lett., 2004, 45, 6875 CrossRef CAS.
- (a) M. Sugiura, K. Hirano and S. Kobayashi, J. Am. Chem. Soc., 2004, 126, 7182 CrossRef CAS PubMed; (b) B. Dhudshia, J. Tiburcio and A. N. Thadani, Chem. Commun., 2005, 5551 RSC.
- (a) J. T. Reeves, Z. Tan, Z. S. Han, G. Li, Y. Zhang, Y. Xu, D. C. Reeves, N. C. Gonnella, S. I. Ma, H. Lee, B. Z. Lu and C. H. Senanayake, Angew. Chem., Int. Ed., 2012, 51, 1400 CrossRef CAS PubMed; (b) T.-L. Liu, C.-J. Wang and X. Zhang, Angew. Chem., Int. Ed., 2013, 52, 8416 CrossRef CAS PubMed.
- (a) J. H. Lee, S. Gupta, W. Jeong, Y. H. Rhee and J. Park, Angew. Chem., Int. Ed., 2012, 51, 10851 CrossRef CAS PubMed; (b) S. Gupta, J. Han, Y. Kim, S. W. Lee, Y. H. Rhee and J. Park, J. Org. Chem., 2014, 79, 9094 CrossRef CAS PubMed; (c) J. Han, M. Jeon, H. K. Pak, Y. H. Rhee and J. Park, Adv. Synth. Catal., 2014, 356, 2769 CrossRef CAS.
- (a) S.-I. Murahashi, Y. Taniguchi, Y. Imada and Y. Tanigawa, J. Org. Chem., 1989, 54, 3292 CrossRef CAS; (b) A. Jabbari, J. Org. Chem., 2010, 1, 6 Search PubMed; (c) A. K. Feldman, B. Colasson, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 13444 CrossRef CAS PubMed.
- E/Z ratios of N–H imines are mentioned in ESI.†.
- M. Rueping, C. Vila and U. Uria, Org. Lett., 2012, 14, 768 CrossRef CAS PubMed.
- Y. Nakao, A. Yada, S. Ebata and T. Hiyama, J. Am. Chem. Soc., 2007, 129, 2428 CrossRef CAS PubMed.
- (a) R. Wada, T. Shibuguchi, S. Makino, K. Oisaki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2006, 128, 7687 CrossRef CAS PubMed; (b) E. M. Vieira, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2011, 133, 3332 CrossRef CAS PubMed.
- (a) L. E. Overman and L. A. Clizbe, J. Am. Chem. Soc., 1976, 98, 2352 CrossRef CAS; (b) J. D. Ha, C. H. Kang, K. A. Belmore and J. K. Cha, J. Org. Chem., 1998, 63, 3810 CrossRef CAS; (c) T. Saito, S. Kobayashi, M. Ohgaki, M. Wada and C. Nagahiro, Tetrahedron Lett., 2002, 43, 2627 CrossRef CAS; (d) F. L. Galbo, E. G. Occhiato, A. Guarna and C. Faggi, J. Org. Chem., 2003, 68, 6360 CrossRef PubMed; (e) S. Kobayashi, T. Furuya, T. Otani and T. Saito, Tetrahedron, 2008, 64, 9705 CrossRef CAS; (f) S. Kobayashi, T. Semba, T. Takahashi, S. Yoshida, K. Dai, T. Otani and T. Saito, Tetrahedron, 2009, 65, 920 CrossRef CAS.
- (a) B. Wu and D. Bai, J. Org. Chem., 1997, 62, 5978 CrossRef CAS; (b) M. Movassaghi, M. Tjandra and J. Qi, J. Am. Chem. Soc., 2009, 131, 9648 CrossRef CAS PubMed; (c) B. B. Liau and M. D. Shair, J. Am. Chem. Soc., 2010, 132, 9594 CrossRef CAS PubMed; (d) D. Fischer, T. X. Nguyen, L. Trzoss, M. Dakanali and E. A. Theodorakis, Tetrahedron Lett., 2011, 52, 4920 CrossRef CAS PubMed; (e) M. G. Vallejo, M. G. Ortega, J. L. Cabrera and A. M. Agnese, Tetrahedron Lett., 2013, 54, 5197 CrossRef CAS.
- Stereochemistry of 9a was assigned by NOE experiments. See ESI.†.
- (a) T. Rizk, E. J.-F. Bilodeau and A. M. Beauchemin, Angew. Chem., Int. Ed., 2009, 48, 8325 CrossRef CAS PubMed; (b) D. A. Colby, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2008, 130, 3645 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26230e |
| This journal is © The Royal Society of Chemistry 2016 |