Enhanced capacitive desalination of MnO2 by forming composite with multi-walled carbon nanotubes†
Bingwei Chena,
Yanfang Wanga,
Zheng Changa,
Xiaowei Wanga,
Minxia Lia,
Xiang Liu*b,
Lixin Zhang*a and
Yuping Wu*ab
aNew Energy and Materials Laboratory (NEML), Department of Chemistry, Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials, Fudan University, Shanghai 200433, China. E-mail: wuyp@fudan.edu.cn; Fax: +86-21-55664223
bCollege of Energy and Institute for Electrochemical Energy Storage, Nanjing Tech University, Nanjing 211816, Jiangsu Province, China
First published on 7th January 2016
Abstract
The morphology and structure of the prepared MnO2/MWCNTs (multi-walled carbon nanotubes) composite are characterized by XRD, SEM, TEM, and N2 sorption analysis. The electrochemical performance of the composite is studied by cyclic voltammetry, electrochemical impedance spectroscopy, and galvanostatic charge/discharge evaluation. The composite has a specific capacitance of 144 F g−1 at the current density of 1 A g−1. It has higher conductivity which is affirmed by electrochemical impedance spectroscopy (EIS). The capacitive deionization (CDI) test was conducted in a bath mode apparatus by assembling a capacitor. The capacitor made from MnO2/MWCNTs composite shows a higher desalination capacity of (6.65 mg g−1) in NaCl aqueous solution, higher than that made from the virginal MnO2 (1.60 mg g−1) and those of the formerly reported. Furthermore, the MnO2/MWCNTs composite electrode shows excellent recyclability with an efficient and rapid regeneration process.
Introduction
Nowadays, due to the population expansion and the increased agricultural activities, many countries over the world are suffering from a shortage of fresh water, which has considerably affected the environment, energy, food, economy and the living standards of people.1 In terms of increasing water supply and managing water demand, seawater desalination is a key technology to enhance both the quantity and quality of water.2 Conventional desalination technologies include multi-effect distillation3 (MED), multi-stage flash4 (MSF), reverse osmosis5 (RO), nuclear desalination6 and electrodialysis7 (ED) technologies. However, these desalination technologies have some problems such as secondary pollution, high cost, maintenance difficulty, and high energy consumption.8 Capacitive desalination technology is new technology which is based on a capacitor and characteristic by low cost and low energy consumption. Specifically, when an external direct voltage (<2 V) is imposed on the electrodes, an electrochemical double layer would be formed in the solution–electrode interface for holding ions. Once the external power is removed or even reversed, the adsorbed ions would be released back to solution resulting in the regeneration.9Basically, the desalination capacity of capacitive deionization (CDI) is mainly governed by the electrode materials. The electrode should has high specific surface area for absorbing ions, good electrical conductivity for decreasing the contact resistance, appropriate pore size, and stable structure for smooth movement of ions and electrolyte wetting.10 Carbon materials such as carbon aerogels,11–13 activated carbon (AC),14–18 graphene,19–21 ordered mesoporous carbon (OMC),22–25 hollow carbon nanofibers,26 and carbon nanotubes (CNTs)27–29 could meet the requirements of CDI electrodes. MnO2 has more advantages among the transition metal oxides such as eco-friendly, low price, good chemical stability and high capacitance, and is widely used in other applications such as supercapacitors and lithium-ion battery.30–34 However, the major drawbacks of MnO2 include its poor electrical conductivity and low specific surface area, which impair their desalination performance. In order to overcome these limitations, an effective approach is to introduce the electronically conductive materials such as CNTs including single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs), which are considered as potential electrode materials for CDI due to their large intrinsic area, good electrical conductivity and chemical stability.35
There are some researches in this area of coating MnO2 on CNTs. A simple chemical bath mode method has been applied to synthesize MnO2/CNT composites, which could achieve 250 F g−1 at 1 A g−1.36 More interestingly, a chemical vapor deposition method can be used to grow CNTs on the stainless steel meshes and then the synthesized MnO2 via a cathodic deposition.37 Furthermore, a three-dimensional MnO2/CNTs composite was prepared by a facile hydrothermal method.38
However, there are few reports on MnO2/CNTs composites for CDI technology. In this paper, we synthesized MnO2/MWCNTs composite and used them as electrode materials for a capacitor in desalination experiment. Results show that the MnO2/MWCNTs composite shows a desalination capacity of 6.65 mg g−1 in 30 mg L−1 NaCl aqueous solution, much higher than those made from the virginal MnO2 (1.60 mg g−1) and the other reported values. In addition, it can meet many CDI electrode requirements such as high desalination efficiency, low cost, and environmental friendly, which provides great promise for practical application of CDI technology.
Experimental
Preparation of composite
The MWCNTs were purchased from Chengdu Organic Chemicals Co. Ltd., Chinese Academy of Science. The activated carbon was purchased from Ningde Xinseng chemical and Industrial Co., Ltd., and used as received, which has a specific surface area of about 1600 m2 g−1. All other chemical reagents in this work were of analytical grade purity from Sinopharm Chemical Reagent Co. Ltd, and used without any purification, and deionized water was used throughout the process. MnO2 was deposited on MWCNTs through a direct redox reaction. Firstly, the MWCNTs with the diameter of 30–50 nm was dispersed in 6 M HNO3 for 1 h with sonication and stirring for another 1 h to remove the impure substances and endow the surface with hydrophilic groups such as –OH and –COOH.39 Then, 200 mg of the acid treated MWCNTs was immersed into an aqueous solution of 100 mL 0.1 M KMnO4 and 100 mL 0.2 M H2SO4 at 85 °C for 1 h. Finally, the mixture was thoroughly filtered with deionization water. The product was dried at 80 °C overnight to obtain MnO2/MWCNTs composite. For comparison, δ-MnO2 was synthesized by using a simple solution method which was shown in ESI.†Characterization
The crystal structures of the samples were characterized using a Bruker D4 X-ray diffractometer with Cu Kα radiation (λ = 1.54056 Å) over a range of 2θ angles from 10° to 90°. The scanning electron microscopic (SEM) measurements were carried out using a Philip XL 300 microscope. A JEOL JEM-2010 transmission electron microscope (TEM) was used to characterize the microstructure of the MnO2/MWCNTs composite. Elemental composition of the composite was measured with energy dispersive spectroscopy (EDS) microanalysis attached to the SEM instrument. Nitrogen adsorption–desorption isotherms were measured with TRISTAR 3000, MICROMERITICS. Specific surface areas were calculated by Brunauer–Emmett–Teller (BET) method. The Barrett–Joyner–Halenda (BJH) model was used to calculate the pore size distributions curve. The thermal behavior of the composite was characterized by the thermal analysis (TG) and differential scanning calorimeter (DSC) analysis (TGA, Du-Pont TGA-2950) at a rising rate of 10 °C min−1 from room temperature to 800 °C in the air atmosphere.Electrochemical tests
The working electrode was prepared by mixing 75 wt% active material, 20 wt% acetylene black and 5 wt% PTFE with the help of some ethanol. Then the mixture was coated onto a stainless steel mesh. After coating, the electrodes were dried at 80 °C overnight. The electrochemical measurements were conducted in a three-electrode electrochemical cell connected to an electrochemical working station of CHI440B at room temperature. The three compartment cell including a MnO2/MWCNTs composite electrode, a stainless steel mesh, and a saturated calomel electrode (SCE), which were used as the working, counter and reference electrodes, respectively. Cyclic voltammetry (CV) tests were carried out in 0.5 M NaCl aqueous solution which is similar to the concentration of the sea water within the potential range from 0 to 1.0 V. The galvanostatic charge/discharge measurements were conducted using an automatic LAND battery test instrument. The charge–discharge performance was evaluated in 0.5 M NaCl aqueous solution. The specific capacitance was calculated according the following equation:
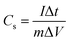 | (1) |
Electrochemical impedance spectroscopy measurements were also measured by CHI440B. The amplitude of the perturbation was 5 mV and the data were collected in the frequency range from 0.01 Hz to 10 kHz.
Desalination experiments
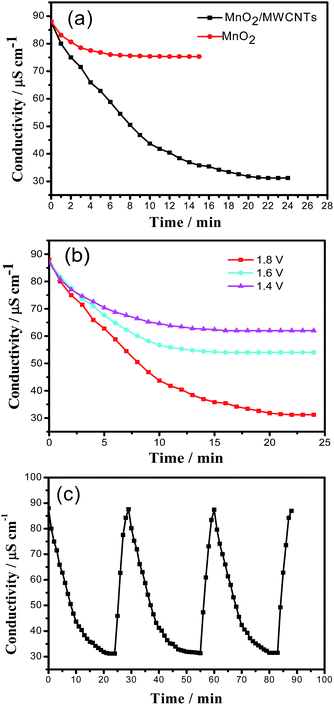
The CDI performance of the electrode was evaluated in a recycle system shown in Fig. 1, including a CDI cell, a peristaltic pump, a direct current power source, a conductivity meter and a pH meter. The electrodes materials were directly attached to the current collector as the CDI electrodes with a total mass of 0.2 g, the MnO2/MWCNTs composite as the cathode and the activated carbon as the anode. In each experiment, the salty solution of 55 mL with an initial conductivity of 87 μS cm−1 was supplied to the CDI cell using a peristaltic pump with a flow rate of 15 mL min−1 and the solution temperature was kept at room temperature. Meanwhile, the amplitude of the applied voltage was 1.8 V. The regeneration of the electrodes was achieved by reversing their polarities. Here the electrosorption capacity (Q) was calculated according the following eqn (2).
 | (2) |
Results and discussion
Morphology and structure
The XRD pattern of the MnO2/MWCNTs composite was shown in Fig. 2a. The MWCNTs show a sharp peak at around 26° corresponding to the crystal plane of (002), and a broad weak peak at around 43° corresponding to that of (100). The MnO2/MWCNTs composite shows another three diffraction peaks at around 12°, 37° and 67° corresponding to the crystal planes of (001), (111) and (020) for δ-MnO2. The intensities of the MnO2 peaks are much lower comparing with those of the peaks of the MWCNTs, indicating their poor crystallinity.Fig. 2b displays the TG and DSC analysis of the composite. The initial weight drop of ∼10 wt% below 200 °C is due to the loss of the water. The following sharp decrease at about 300 °C is originated from the oxidation of MWCNTs in the air. Based on the TG analysis, the MnO2 content is estimated to be 78 wt% in the composite.
As shown in Fig. 3a, the acid-treated MWCNTs display that its diameter is about 30–50 nm. In Fig. 3b, the MnO2 exhibits the morphology of nanoflake. From the SEM (Fig. 3c) and TEM (Fig. 3d) micrographs of the composite, it can be seen that the MnO2 is nanoflake-like (30–50 nm in thickness), and MnO2 nanoflakes are fully grown on the surface of the MWCNTs. In addition, energy dispersive X-ray spectroscopy (EDX) analysis in Fig. 3e indicates the presence of C, Mn and O elements, which is consistent with the composite of MnO2 with CNTs.
 | ||
| Fig. 3 SEM micrographs of (a) MWCNTs, (c) MnO2/MWCNTs composite, TEM micrograph of (b) pristine MnO2 and (d) MnO2/MWCNTs composite and corresponding EDX analysis (e). | ||
The N2 adsorption–desorption isotherms and the pore size distribution of the samples are shown in Fig. 4. The parameters of the surface area and porosity were calculated and listed in Table 1. It is clearly observed that the two samples display an adsorption of type IV with a small hysteresis loop of H3 and the pore size distribution indicates the presence of mesoporous in these porous structures. The MnO2/MWCNTs composite displays a high specific surface area of 134 m2 g−1 with a pore volume of 0.664 cm3 g−1, which is much higher than the virginal MnO2 (specific surface area of 7.61 m2 g−1 and a pore volume of 0.0347 cm3 g−1). This may be resulted from the addition of the MWCNTs. As to the detailed reason, it needs further investigation. The increased specific surface area and pore volume of the MnO2/MWCNTs composite can ensure more accessible surface sites for ion adsorption during the CDI process.
 | ||
| Fig. 4 N2 adsorption–desorption isotherms and pore size distribution (inset) of (a) the MnO2/MWCNTs composite and (b) the virginal MnO2. | ||
| Sample | SBET (m2 g−1) | Vtot (cm3 g−1) | Dav (nm) |
|---|---|---|---|
| MnO2/MWCNTs | 134 | 0.664 | 19.7 |
| MnO2 | 7.61 | 0.0347 | 18.45 |
Electrochemical behaviour
Fig. 5a displays the CV curves of the MnO2/MWCNTs composite and the virginal MnO2 electrodes in 0.5 M NaCl aqueous solution at a scan rate of 1 mV s−1 in a potential range of 0 to 1.0 V. Both the CV curves show a nearly rectangular shape with a pair of weak redox peaks at about 0.5–0.7 V, indicating the pseudocapacitance from redox reactions between Mn(IV) and Mn(III) or Mn(II).40 Moreover, the specific capacitance calculated according to eqn (1) from the CV curves is 163 F g−1 for the MnO2/MWCNTs composite which is much higher than the virginal MnO2 (119 F g−1). The main reason is that the MWCNTs can not only improve the surface area of MnO2 but also decrease the resistance of the MnO2/MWCNTs composite. | ||
| Fig. 5 (a) CV curves for two electrodes at scan rate of 1 mV s−1, (b) the MnO2/MWCNTs composite at different scan rates in 0.5 M NaCl aqueous solution. | ||
Fig. 5b shows the CV curves of the MnO2/WMCNTs composite in 0.5 M NaCl aqueous solution at different scan rates. It can be observed that at low scan rates, the electrode shows rectangular shape. With the increase of the scan rate, the shape of rectangle is slightly distorted due to the polarization and the resistance of the electrodes.41 The specific capacitances of the MnO2/MWCNTs composite are 163, 155, 144, 137, 121, 110, 93, 66 and 35 F g−1 at the scan rates of 1, 2, 5, 10, 20, 30, 50, 100 and 200 mV s−1, respectively. Obviously, the capacitance decreases with the increase of the scan rate. This is because at high scan rate, the ions do not have enough time to diffuse into all the available pores of the MnO2 and some internal active MnO2 species cannot be used, which causes lower specific capacitance.16
The Nyquist plots for the MnO2/MWCNTs composite and the virginal MnO2 are shown in Fig. 6a. The alternative current impedance spectra for both of them compose of a semicircle in the high-to-medium-frequency region, and a straight line at the very low-frequency region. The semicircle corresponding to a parallel combination of charge-transfer resistance (Rct) and MnO2/MWCNTs composite is smaller than that of the MnO2, indicating that the MWCNTs can decrease the Rct of the MnO2/MWCNTs composite. The intercepts on the real Z′ axis shows the equivalent series resistance (ESR) values for the electrolytes. The MnO2/MWCNTs composite has a lower ESR value compare to the MnO2, which refer to a lower contact resistance at the interface between the active material and the aqueous solution.42
Fig. 6b shows the constant-current charge/discharge curve of the MnO2/MWCNTs composite at different current density in 0.5 M NaCl aqueous solution. According to eqn (1), the specific capacitance of the composite is 151 F g−1 at low current density of 0.25 A g−1. As the current density increases to 2 A g−1, the specific capacitance remains at 123 F g−1, less than 19% capacity fading which indicates the excellent rate capability. The charge/discharge curves of the MnO2 and the MnO2/MWCNTs composite in 0.5 M NaCl aqueous solution at 1 A g−1 is shown in Fig. 6c. The discharge time of the MnO2/MWCNTs composite electrode is longer than that of the MnO2. The specific capacitance calculated according to eqn (1) is 144 F g−1 for the MnO2/MWCNTs composite which is much higher than the virginal MnO2 (109 F g−1). In addition, the IR drop of the MnO2/MWCNTs composite is smaller than that of the MnO2 since the MnO2/MWCNTs composite has lower internal resistance. The cycling performance of the MnO2/MWCNTs composite at the current density of 1 A g−1 (Fig. 6d) shows that the coulombic efficiency is almost 100% and it loses less than 14% of the initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. This result indicates excellent cycling stability and capacity retention in NaCl even oxygen is not removed.
000 cycles. This result indicates excellent cycling stability and capacity retention in NaCl even oxygen is not removed.
CDI tests
To estimate the desalination performance of the fabricated supercapacitor, the bath mode experiments were conducted in a 30 ppm NaCl aqueous solution that has an initial conductivity of 87 μS cm−1 at an applied voltage of 1.8 V. Fig. 7a gives the desalination curves of the cells based on the MnO2/MWCNTs composite and the virginal MnO2. A dramatic decrease of the conductivity appears for both cells at the beginning stage which indicates quick adsorption of the salt ions. In the second stage, the conductivity tends to get constant due to the electrosorption saturation. The electrosorption capacity of the MnO2/MWCNTs composite cell was calculated according to eqn (2) as 6.65 mg g−1, which is much higher than that of the cell based on the virginal MnO2 (1.60 mg g−1), and exceed those of the reports about CDI electrode materials to our knowledge, which are listed in Table 2. The superior desalination capacity of the MnO2/MWCNTs composite is mainly due to the high specific capacitance, good conductivity, large specific surface area and suitable pore structure. Fig. 7b shows the effect of applied voltage on the desalination performance. The experiments were conducted at various voltages of 1.4, 1.6, 1.8 V in 0.03 g L−1 NaCl solution. It can be seen that the higher the applied voltage, the higher the desalting capacity is. When the applied voltage increases from 1.4, 1.6, to 1.8 V, the electrosorption capacity reaches 2.93, 3.87, 6.65 mg g−1, respectively.| Sample | Initial concentration (mg L−1) | Electrosorption capacity (mg g−1) | Ref. |
|---|---|---|---|
| a Note: MC for mesoporous carbon, NPC for nanoporous carbon, and GNS for graphene nanosheet. | |||
| AC | ∼25 | 0.25 | 43 |
| CNTs | 60 | 0.7 | 44 |
| CNTs–MC | 40 | 0.63 | 45 |
| MnO2/NPC | 50 | 0.988 | 10 |
| AC–MnO2 | ∼25 | 0.99 | 46 |
| GNS–MnO2 | 100 | 5.01 | 42 |
| MnO2@MWCNTs | ∼87 | 6.65 | This work |
Fig. 7c displays the repeated performance of the MnO2/MWCNTs composite electrode. The electrosorption capacity of NaCl for the cell based on the MnO2/MWCNTs composite during the three sequent runs is 6.65, 6.59, and 6.56 mg g−1, respectively. In addition, the MnO2/MWCNTs composite electrode has no appreciable decrease in the initial conductivity.
During the desalination and regeneration progress, the pH values of the solution remained stable within a range of 5.6–6.0, ensuring no hydrolysis of water.
The CDI results suggest that the MnO2/MWCNTs composite performs very well with high desalination efficiency. Therefore, the composite has a great potential as the electrode material for CDI technology.
Conclusion
In this paper, a MnO2/MWCNTs composite was successfully synthesized through a facile and green method, and used as a potential application as an electrode of CDI device. The MWCNTs are fully and uniformly coated with MnO2. The N2 sorption analysis demonstrates that the MnO2/MWCNTs composite has a larger specific surface area as compared with the virginal MnO2. It is noted that the surface area is a decisive factor influencing the desalination capacitance.47 In all the electrochemical evaluation, the MnO2/MWCNTs composite exhibits superior electrochemical properties as compared with the virginal MnO2, which is due to the larger surface area and higher electronic conductivity. Furthermore, the desalination experiments demonstrate that the removal of ions from a NaCl aqueous solution can be successfully achieved by using the MnO2/MWCNTs composite electrodes. The electrosorptive capacity of the MnO2/MWCNTs composite and MnO2 is 6.65 and 1.60 mg g−1, respectively. By comparing with the virginal MnO2 electrode, the MnO2/MWCNTs composite electrode shows larger electrosorption capacity and good repeated performance during the CDI process. Therefore, the MnO2/MWCNTs composite can be considered as a promising material for CDI technology.Acknowledgements
Financial support from rom China National Distinguished Youth Scientists (NSFC No. 51425301) is greatly appreciated.Notes and references
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marin and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed
.
- R. F. Service, Science, 2006, 313, 1088–1090 CrossRef CAS PubMed
.
- A. Hamidi, K. Parham, U. Atikol and A. H. Shahbaz, Desalination, 2015, 371, 37–45 CrossRef CAS
.
- M. M. Alhazmy, Energy, 2014, 76, 1029–1035 CrossRef
.
- M. S. Japas, N. A. Rubinstein and A. L. R. Gómez, Ore Geol. Rev., 2015, 71, 191–202 CrossRef
.
- A. P. Avrin, G. He and D. M. Kammen, Desalination, 2015, 360, 1–7 CrossRef CAS
.
- X. P. Zhu, W. H. He and B. E. Logan, J. Membr. Sci., 2015, 494, 154–160 CrossRef CAS
.
- X. R. Wen, D. S. Zhang, T. T. Yan, J. P. Zhang and L. Y. Shi, J. Mater. Chem. A, 2013, 1, 12334–12344 CAS
.
- Z. Wang, B. J. Dou, L. Zheng, G. N. Zhang, Z. H. Liu and Z. P. Hao, Desalination, 2012, 299, 96–102 CrossRef CAS
.
- J. Yang, L. Zou, H. H. Song and Z. P. Hao, Desalination, 2011, 276, 199–206 CrossRef CAS
.
- Y. F. Zhang, W. Fan, Y. P. Huang, C. Zhang and T. X. Liu, RSC Adv., 2015, 5, 1301–1308 RSC
.
- A. Allahbakhsh and A. R. Bahramian, Nanoscale, 2015, 5, 14139–14158 RSC
.
- S.-A. Wohlgemuth, T.-P. Fellinger, P. Jaker and M. Antonietti, J. Mater. Chem. A, 2013, 1, 4002–4009 CAS
.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250–1280 CAS
.
- P. Cheng, S. Y. Gao, P. Y. Zang, X. F. Yang, Y. L. Bai, H. Xu, Z. H. Liu and Z. B. Lei, Carbon, 2015, 93, 315–324 CrossRef CAS
.
- D. B. Wang, Z. Geng, B. Li and C. M. Zhang, Electrochim. Acta, 2015, 173, 377–384 CrossRef CAS
.
- E. J. Lee, Y. J. Lee, J. K. Kim, M. Lee, J. Yi, J. R. Yoon, J. C. Song and I. K. Song, Mater. Res. Bull., 2015, 70, 209–214 CrossRef CAS
.
- Y. Gao, L. Li, Y. M. Jin, Y. Wang, C. J. Yuan, Y. J. Wei, G. Chen, J. J. Ge and H. Y. Lu, Appl. Energy, 2015, 153, 41–77 CrossRef CAS
.
- S. P. Wu, R. Xu, M. J. Lu, R. Y. Ge, J. Iocozzia, C. P. Han, B. B. Jiang and Z. Q. Lin, Adv. Energy Mater., 2015, 5, 1500400 Search PubMed
.
- X. L. Wang and G. Q. Shi, Energy Environ. Sci., 2015, 8, 790–823 CAS
.
- V. Meriga, S. Valligatla, S. Sundaresan, C. Cahill, V. R. Dhanak and A. K. Chakraborty, J. Appl. Polym. Sci., 2015, 42766, 1–9 Search PubMed
.
- Z. J. Zhu, Y. J. Hu, H. Jiang and C. Z. Li, J. Power Sources, 2014, 246, 402–408 CrossRef CAS
.
- J. K. Hu, M. Noked, E. Gillette, Z. Gui and S. B. Lee, Carbon, 2015, 93, 903–914 CrossRef CAS
.
- M. Enterría, M. F. R. Pereira, J. I. Martins and J. L. Figueiredo, Carbon, 2015, 95, 72–83 CrossRef
.
- S. Tanaka, H. Fujimoto, J. F. M. Denayer, M. Miyamoto, Y. Oumi and Y. Miyake, Microporous Mesoporous Mater., 2015, 217, 141–149 CrossRef CAS
.
- A. G. El-Deen, N. A. M. Barakat, K. A. Khalild and H. Y. Kim, New J. Chem., 2014, 38, 198–205 RSC
.
- H. Y. Liu, H. H. Song, X. H. Chen, S. Zhang, J. S. Zhou and Z. K. Ma, J. Power Sources, 2015, 285, 303–309 CrossRef CAS
.
- M. Li, F. Liu, J. P. Cheng, J. Ying and X. B. Zhang, J. Alloys Compd., 2015, 635, 225–232 CrossRef CAS
.
- T.-T. Lin, W.-H. Lai, Q.-F. Lü and Y. Yu, Electrochim. Acta, 2015, 178, 517–524 CrossRef CAS
.
- X. W. Guo, J. H. Han, L. Zhang, P. Liu, A. Hirata, L. Y. Chen, T. Fujitaa and M. W. Chen, Nanoscale, 2015, 7, 15111–15116 RSC
.
- C. X. Guo, A. A. Chitre and X. M. Lu, Phys. Chem. Chem. Phys., 2014, 16, 4672–4678 RSC
.
- D. J. Wu, S. H. Xu, M. Li, C. Zhang, Y. P. Zhu, Y. W. Xu, W. W. Zhang, R. Huang, R. J. Qi, L. W. Wang and P. K. Chu, J. Mater. Chem. A, 2015, 3, 16695–16707 CAS
.
- M. Huang, F. Li, F. Dong, Y. X. Zhang and L. L. Zhang, Prog. Mater. Sci., 2015, 74, 51–124 CrossRef
.
- J. Zhang, Y. P. Luan, Z. Y. Lyu, L. J. Wang, L. L. Xu, K. Yuan, F. Pan, M. Lai, Z. L. Liu and W. Chen, Nanoscale, 2015, 7, 14881–14888 RSC
.
- H. J. Wang, C. Peng, F. Peng, H. Yu and J. Yang, Mater. Sci. Eng., B, 2011, 176, 1073–1078 CrossRef CAS
.
- S.-B. Ma, K.-W. Nam, W.-S. Yoon, X.-Q. Yang, K.-Y. Ahn, K.-H. Ohd and K.-B. Kim, J. Power Sources, 2008, 178, 483–489 CrossRef CAS
.
- Y. H. Wang, H. Liu, X. L. Sunb and I. Zhitomirsky, Scr. Mater., 2009, 61, 1079–1082 CrossRef CAS
.
- F. Teng, S. Santhanagopalan, Y. Wang and D. D. Meng, J. Alloys Compd., 2010, 499, 259–264 CrossRef CAS
.
- W. Tang, Y. Y Hou, F. X. Wang, L. L. Liu, Y. P. Wu and K. Zhu, Nano Lett., 2013, 13, 2036–2040 CrossRef CAS PubMed
.
- Q. T. Qu, P. Zhang, B. Wang, Y. H. Chen, S. Tian, Y. P. Wu and R. Holze, J. Phys. Chem. C, 2009, 113, 14020–14027 CAS
.
- J. Yan, T. Wei, Z. J. Fan, W. Z. Qian, M. L. Zhang, X. D. Shen and F. Wei, J. Power Sources, 2010, 195, 3041–3045 CrossRef CAS
.
- A. G. El-Deen, N. A. M. Barakat and H. Y. Kim, Desalination, 2014, 344, 289–298 CrossRef CAS
.
- L. Zou, L. X. Li, H. H. Song and G. Morris, Water Res., 2008, 42, 2340–2348 CrossRef CAS PubMed
.
- M. Wang, Z. H. Huang, L. Wang, M.-X. Wang, F. Y. Kang and H. Q. Hou, New J. Chem., 2010, 34, 1843–1845 RSC
.
- D. S. Zhang, L. Y. Shi, J. H. Fang and K. Dai, Mater. Lett., 2006, 60, 360–363 CrossRef CAS
.
- J. Yang, L. Zou, H. H. Song and Z. P. Hao, Desalination, 2012, 286, 108–114 CrossRef CAS
.
- M. T. Z. Myint, S. H. Al-Harthi and J. Dutta, Desalination, 2014, 344, 236–242 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26210k |
| This journal is © The Royal Society of Chemistry 2016 |