Lipid membrane formation on chemical gradient modified surfaces†
Abstract
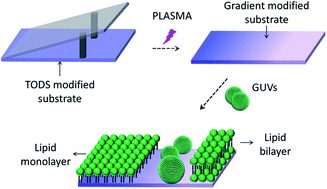
The relationship between surface wetting properties and lipid membrane status formed via giant unilamellar vesicle rupture was investigated using chemical gradient surfaces. Fluorescence microscopy and AFM analysis confirmed that GUVs could form uniform monolayers, monolayer patches and bilayer patches on surface regions with contact angles ranging from 108° to ∼61°, ∼60° to ∼55° and less than 5°, respectively. The intact GUVs stand in the area with contact angle between ∼54° and ∼28°.

 Please wait while we load your content...
Please wait while we load your content...